Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation
- PMID: 15175265
- PMCID: PMC420359
- DOI: 10.1101/gad.1184904
Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation
Abstract
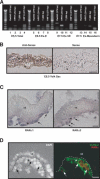
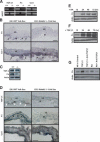
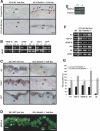
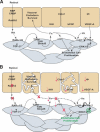
We previously demonstrated that during vascular morphogenesis, retinoic acid (RA) is required for the control of endothelial cell proliferation and capillary plexus remodeling. Herein, we investigate the mechanisms by which RA regulates these processes in the yolk sac. We found that although the enzyme required for RA production during early embryogenesis, retinaldehyde dehydrogenase-2 (Raldh2), was expressed in the visceral endoderm, RA receptors alpha1 and alpha2 were expressed in endothelial cells in the mesoderm, indicating that they are direct targets of RA. In Raldh2(-/-) embryos, there was down-regulation of TGF-beta1, fibronectin (Fn) and integrin alpha5, which was associated with decreased visceral endoderm survival and production of VEGF-A, Indian hedgehog (IHH), and bFGF. Exogenous provision of RA or Fn to Raldh2(-/-) explants in whole mouse embryo culture restored vascular remodeling, visceral endoderm survival, as well as integrin alpha5 expression and its downstream signaling that controls endothelial growth. Exogenous provision of visceral endoderm-derived factors (VEGF-A, IHH, and bFGF) failed to rescue endothelial cell proliferative control but collectively promoted vascular remodeling, suggesting that these processes are independently regulated via a signaling hierarchy downstream of RA.
Figures




References
-
- Akhurst, R.J., Lehnert, S.A., Faissner, A.J., and Duffie, E. 1990. Tgf β in murine morphogenetic processes: The early embryo and cardiogenesis. Development 108: 645-656. - PubMed
-
- Baldwin, H.S. 1996. Early embryonic vascular development. Cardiovasc. Res. 31: E34-E45. - PubMed
-
- Baron, M.H. 2001. Induction of embryonic hematopoietic and endothelial stem/progenitor cells by hedgehog-mediated signals. Differentiation 68: 175-185. - PubMed
-
- Bavik, C.O., Peterson, P., and Eriksson, U. 1995. Retinol-binding protein mediates uptake of retinol to cultured human keratinocytes. Exp. Cell. Res. 216: 358-362. - PubMed
-
- Bavik, C.O., Ward, S.J., and Ong, D.E. 1997. Identification of a mechanism to localize generation of retinoic acid in rat embryos. Mech. Dev. 69: 155-167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
