Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein
- PMID: 15175286
- PMCID: PMC419962
- DOI: 10.1128/JB.186.12.3730-3737.2004
Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein
Abstract
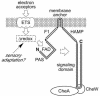
Aer is a membrane-associated protein that mediates aerotactic responses in Escherichia coli. Its C-terminal half closely resembles the signaling domains of methyl-accepting chemotaxis proteins (MCPs), which undergo reversible methylation at specific glutamic acid residues to adapt their signaling outputs to homogeneous chemical environments. MCP-mediated behaviors are dependent on two specific enzymes, CheR (methyltransferase) and CheB (methylesterase). The Aer signaling domain contains unorthodox methylation sites that do not conform to the consensus motif for CheR or CheB substrates, suggesting that Aer, unlike conventional MCPs, might be a methylation-independent transducer. Several lines of evidence supported this possibility. (i) The Aer protein was not detectably modified by either CheR or CheB. (ii) Amino acid replacements at the putative Aer methylation sites generally had no deleterious effect on Aer function. (iii) Aer promoted aerotactic migrations on semisolid media in strains that lacked all four of the E. coli MCPs. CheR and CheB function had no influence on the rate of aerotactic movements in those strains. Thus, Aer senses and signals efficiently in the absence of deamidation or methylation, methylation changes, methylation enzymes, and methyl-accepting chemotaxis proteins. We also found that chimeric transducers containing the PAS-HAMP sensing domain of Aer joined to the signaling domain and methylation sites of Tar, an orthodox MCP, exhibited both methylation-dependent and methylation-independent aerotactic behavior. The hybrid Aear transducers demonstrate that methylation independence does not emanate from the Aer signaling domain but rather may be due to transience of the cellular redox changes that are thought to trigger Aer-mediated behavioral responses.
Figures







Comment in
-
An alternative strategy for adaptation in bacterial behavior.J Bacteriol. 2004 Jun;186(12):3671-3. doi: 10.1128/JB.186.12.3671-3673.2004. J Bacteriol. 2004. PMID: 15175278 Free PMC article. No abstract available.
Similar articles
-
Interactions between the PAS and HAMP domains of the Escherichia coli aerotaxis receptor Aer.J Bacteriol. 2004 Nov;186(21):7440-9. doi: 10.1128/JB.186.21.7440-7449.2004. J Bacteriol. 2004. PMID: 15489456 Free PMC article.
-
Signaling interactions between the aerotaxis transducer Aer and heterologous chemoreceptors in Escherichia coli.J Bacteriol. 2006 May;188(10):3487-93. doi: 10.1128/JB.188.10.3487-3493.2006. J Bacteriol. 2006. PMID: 16672602 Free PMC article.
-
Inversion of aerotactic response in Escherichia coli deficient in cheB protein methylesterase.J Bacteriol. 1986 Apr;166(1):275-80. doi: 10.1128/jb.166.1.275-280.1986. J Bacteriol. 1986. PMID: 3007436 Free PMC article.
-
Aer on the inside looking out: paradigm for a PAS-HAMP role in sensing oxygen, redox and energy.Mol Microbiol. 2007 Sep;65(6):1415-24. doi: 10.1111/j.1365-2958.2007.05889.x. Mol Microbiol. 2007. PMID: 17824925 Free PMC article. Review.
-
Aerotaxis and other energy-sensing behavior in bacteria.Annu Rev Microbiol. 1999;53:103-28. doi: 10.1146/annurev.micro.53.1.103. Annu Rev Microbiol. 1999. PMID: 10547687 Review.
Cited by
-
Conformational suppression of inter-receptor signaling defects.Proc Natl Acad Sci U S A. 2006 Jun 13;103(24):9292-7. doi: 10.1073/pnas.0602135103. Epub 2006 Jun 2. Proc Natl Acad Sci U S A. 2006. PMID: 16751275 Free PMC article.
-
Biphasic control logic of HAMP domain signalling in the Escherichia coli serine chemoreceptor.Mol Microbiol. 2011 May;80(3):596-611. doi: 10.1111/j.1365-2958.2011.07577.x. Epub 2011 Feb 24. Mol Microbiol. 2011. PMID: 21306449 Free PMC article.
-
A Single-cell genome for Thiovulum sp.Appl Environ Microbiol. 2012 Dec;78(24):8555-63. doi: 10.1128/AEM.02314-12. Epub 2012 Sep 28. Appl Environ Microbiol. 2012. PMID: 23023751 Free PMC article.
-
Aer Receptors Influence the Pseudomonas chlororaphis PCL1606 Lifestyle.Front Microbiol. 2020 Jul 8;11:1560. doi: 10.3389/fmicb.2020.01560. eCollection 2020. Front Microbiol. 2020. PMID: 32754135 Free PMC article.
-
Role of CheB and CheR in the complex chemotactic and aerotactic pathway of Azospirillum brasilense.J Bacteriol. 2006 Jul;188(13):4759-68. doi: 10.1128/JB.00267-06. J Bacteriol. 2006. PMID: 16788185 Free PMC article.
References
-
- Alexeyev, M. F., I. N. Shokolenko, and T. P. Croughan. 1995. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene 160:63-67. - PubMed
-
- Ames, P., and J. S. Parkinson. 1988. Transmembrane signaling by bacterial chemoreceptors: E. coli transducers with locked signal output. Cell 55:817-826. - PubMed
-
- Ames, P., Y. A. Yu, and J. S. Parkinson. 1996. Methylation segments are not required for chemotactic signalling by cytoplasmic fragments of Tsr, the methyl-accepting serine chemoreceptor of Escherichia coli. Mol. Microbiol. 19:737-746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

