Mutational analysis of transmembrane regions 3 and 4 of SecY, a central component of protein translocase
- PMID: 15175310
- PMCID: PMC419966
- DOI: 10.1128/JB.186.12.3960-3969.2004
Mutational analysis of transmembrane regions 3 and 4 of SecY, a central component of protein translocase
Abstract
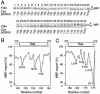
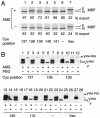
The SecYEG heterotrimeric membrane protein complex functions as a channel for protein translocation across the Escherichia coli cytoplasmic membrane. SecY is the central subunit of the SecYEG complex and contains 10 transmembrane segments (TM1 to TM10). Previous mutation studies suggested that TM3 and TM4 are particularly important for SecY function. To further characterize TM3 and TM4, we introduced a series of cysteine-scanning mutations into these segments. With one exception (an unstable product), all the mutant proteins complemented the cold-sensitive growth defect of the secY39 mutant. A combination of this secY mutation and the secG deletion resulted in synthetic lethality, and the TM3 and TM4 SecY cysteine substitution mutations were examined for their ability to complement this lethality. Although they were all positive for complementation, some of the complemented cells exhibited significant retardation of protein export. The substitution-sensitive residues in TM3 can be aligned to one side of the alpha-helix, and those in TM4 revealed a tendency for residues closer to the cytosolic side of the membrane to be more severely affected. Disulfide cross-linking experiments identified a specific contact point for TM3 and SecG TM2 as well as for TM4 and SecG TM1. Thus, although TM3 and TM4 do not contain any single residue that is absolutely required, they include functionally important helix surfaces and specific contact points with SecG. These results are discussed in light of the structural information available for the SecY complex.
Figures
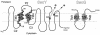



Similar articles
-
Oligomeric states of the SecA and SecYEG core components of the bacterial Sec translocon.Biochim Biophys Acta. 2007 Jan;1768(1):5-12. doi: 10.1016/j.bbamem.2006.08.013. Epub 2006 Aug 30. Biochim Biophys Acta. 2007. PMID: 17011510 Free PMC article. Review.
-
Nearest neighbor analysis of the SecYEG complex. 1. Identification of a SecY-SecG interface.Biochemistry. 2003 Jun 24;42(24):7434-41. doi: 10.1021/bi034331a. Biochemistry. 2003. PMID: 12809499
-
Membrane topology inversion of SecG detected by labeling with a membrane-impermeable sulfhydryl reagent that causes a close association of SecG with SecA.J Biochem. 2002 Oct;132(4):629-34. doi: 10.1093/oxfordjournals.jbchem.a003266. J Biochem. 2002. PMID: 12359079
-
In vivo cross-linking of the SecA and SecY subunits of the Escherichia coli preprotein translocase.J Bacteriol. 1997 Sep;179(18):5699-704. doi: 10.1128/jb.179.18.5699-5704.1997. J Bacteriol. 1997. PMID: 9294424 Free PMC article.
-
The Sec system.Curr Opin Microbiol. 1998 Apr;1(2):216-22. doi: 10.1016/s1369-5274(98)80014-3. Curr Opin Microbiol. 1998. PMID: 10066476 Review.
Cited by
-
Oligomeric states of the SecA and SecYEG core components of the bacterial Sec translocon.Biochim Biophys Acta. 2007 Jan;1768(1):5-12. doi: 10.1016/j.bbamem.2006.08.013. Epub 2006 Aug 30. Biochim Biophys Acta. 2007. PMID: 17011510 Free PMC article. Review.
-
The bacterial Sec-translocase: structure and mechanism.Philos Trans R Soc Lond B Biol Sci. 2012 Apr 19;367(1592):1016-28. doi: 10.1098/rstb.2011.0201. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22411975 Free PMC article. Review.
-
Redox-active cysteines of a membrane electron transporter DsbD show dual compartment accessibility.EMBO J. 2007 Aug 8;26(15):3509-20. doi: 10.1038/sj.emboj.7601799. Epub 2007 Jul 19. EMBO J. 2007. PMID: 17641688 Free PMC article.
-
Different modes of SecY-SecA interactions revealed by site-directed in vivo photo-cross-linking.Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16159-64. doi: 10.1073/pnas.0606390103. Epub 2006 Oct 23. Proc Natl Acad Sci U S A. 2006. PMID: 17060619 Free PMC article.
-
SecY alterations that impair membrane protein folding and generate a membrane stress.J Cell Biol. 2007 Jan 29;176(3):307-17. doi: 10.1083/jcb.200611121. Epub 2007 Jan 22. J Cell Biol. 2007. PMID: 17242069 Free PMC article.
References
-
- Breyton, C., W. Haase, T. A. Rapoport, W. Kuhlbrandt, and I. Collinson. 2002. Three-dimensional structure of the bacterial protein-translocation complex SecYEG. Nature 418:662-665. - PubMed
-
- Economou, A., and W. Wickner. 1994. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell 78:835-843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

