Involvement of the SppA1 peptidase in acclimation to saturating light intensities in Synechocystis sp. strain PCC 6803
- PMID: 15175313
- PMCID: PMC419952
- DOI: 10.1128/JB.186.12.3991-3999.2004
Involvement of the SppA1 peptidase in acclimation to saturating light intensities in Synechocystis sp. strain PCC 6803
Abstract
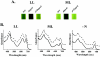
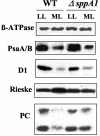
The sll1703 gene, encoding an Arabidopsis homologue of the thylakoid membrane-associated SppA peptidase, was inactivated by interposon mutagenesis in Synechocystis sp. strain PCC 6803. Upon acclimation from a light intensity of 50 to 150 microE m(-2) s(-1), the mutant preserved most of its phycobilisome content, whereas the wild-type strain developed a bleaching phenotype due to the loss of about 40% of its phycobiliproteins. Using in vivo and in vitro experiments, we demonstrate that the DeltasppA1 strain does not undergo the cleavage of the L(R)(33) and L(CM)(99) linker proteins that develops in the wild type exposed to increasing light intensities. We conclude that a major contribution to light acclimation under a moderate light regime in cyanobacteria originates from an SppA1-mediated cleavage of phycobilisome linker proteins. Together with changes in gene expression of the major phycobiliproteins, it contributes an additional mechanism aimed at reducing the content in phycobilisome antennae upon acclimation to a higher light intensity.
Figures
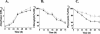
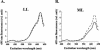



Similar articles
-
Synechocystis sp PCC 6803 strains lacking photosystem I and phycobilisome function.Plant Cell. 1993 Dec;5(12):1853-63. doi: 10.1105/tpc.5.12.1853. Plant Cell. 1993. PMID: 8305875 Free PMC article.
-
Characterization of Synechocystis sp. strain PCC 6803 and deltanbl mutants under nitrogen-deficient conditions.Arch Microbiol. 2002 Oct;178(4):256-66. doi: 10.1007/s00203-002-0446-y. Epub 2002 Jun 29. Arch Microbiol. 2002. PMID: 12209258
-
Structural and compositional analyses of the phycobilisomes of Synechococcus sp. PCC 7002. Analyses of the wild-type strain and a phycocyanin-less mutant constructed by interposon mutagenesis.Arch Microbiol. 1990;153(6):550-60. doi: 10.1007/BF00245264. Arch Microbiol. 1990. PMID: 2164365
-
The phycobilisome, a light-harvesting complex responsive to environmental conditions.Microbiol Rev. 1993 Sep;57(3):725-49. doi: 10.1128/mr.57.3.725-749.1993. Microbiol Rev. 1993. PMID: 8246846 Free PMC article. Review.
-
Acclimation to high-light conditions in cyanobacteria: from gene expression to physiological responses.J Plant Res. 2012 Jan;125(1):11-39. doi: 10.1007/s10265-011-0454-6. Epub 2011 Oct 18. J Plant Res. 2012. PMID: 22006212 Review.
Cited by
-
Light Modulation for Bioactive Pigment Production in Synechocystis salina.Bioengineering (Basel). 2022 Jul 21;9(7):331. doi: 10.3390/bioengineering9070331. Bioengineering (Basel). 2022. PMID: 35877382 Free PMC article.
-
Loss of chloroplast protease SPPA function alters high light acclimation processes in Arabidopsis thaliana L. (Heynh.).J Exp Bot. 2009;60(6):1715-27. doi: 10.1093/jxb/erp051. Epub 2009 Apr 6. J Exp Bot. 2009. PMID: 19349419 Free PMC article.
-
A bioelectrochemical approach to characterize extracellular electron transfer by Synechocystis sp. PCC6803.PLoS One. 2014 Mar 17;9(3):e91484. doi: 10.1371/journal.pone.0091484. eCollection 2014. PLoS One. 2014. PMID: 24637387 Free PMC article.
-
Structure, function, and assembly of PSI in thylakoid membranes of vascular plants.Plant Cell. 2024 Oct 3;36(10):4080-4108. doi: 10.1093/plcell/koae169. Plant Cell. 2024. PMID: 38848316 Free PMC article. Review.
-
A proteomic analysis of the chromoplasts isolated from sweet orange fruits [Citrus sinensis (L.) Osbeck].J Exp Bot. 2011 Nov;62(15):5297-309. doi: 10.1093/jxb/err140. Epub 2011 Aug 12. J Exp Bot. 2011. PMID: 21841170 Free PMC article.
References
-
- Anderson, J. M., W. S. Chow, and Y. I. Park. 1995. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth. Res. 46:129-139. - PubMed
-
- Arnon, D. I., B. D. McSwain, H. Y. Tsujimoto, and K. Wad. 1974. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim. Biophys. Acta 357:231-245. - PubMed
-
- Bolhuis, A., A. Matzen, H. L. Hyyrylainen, V. P. Kontinen, R. Meima, J. Chapuis, G. Venema, S. Bron, R. Freudl, and J. M. van Dijl. 1999. Signal peptide peptidase- and ClpP-like proteins of Bacillus subtilis required for efficient translocation and processing of secretory proteins. J. Biol. Chem. 274:24585-24592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

