Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent
- PMID: 15175377
- PMCID: PMC6729199
- DOI: 10.1523/JNEUROSCI.0882-04.2004
Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent
Abstract
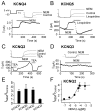
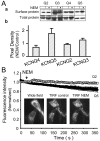
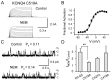
The cysteine-modifying reagent N-ethylmaleimide (NEM) is known to augment currents from native M-channels in sympathetic neurons and cloned KCNQ2 channels. As a probe for channel function, we investigated the mechanism of NEM action and subunit specificity of cloned KCNQ2-5 channels expressed in Chinese hamster ovary cells at the whole-cell and single-channel levels. Biotinylation assays and total internal reflection fluorescence microscopy indicated that NEM action is not caused by increased trafficking of channels to the membrane. At saturating voltages, whole-cell currents of KCNQ2, KCNQ4, and KCNQ5 but not KCNQ3 were augmented threefold to fourfold by 50 microm NEM, and their voltage dependencies were negatively shifted by 10-20 mV. Unitary conductances of KCNQ2 and KCNQ3 (6.2 and 8.5 pS, respectively) were much higher that those of KCNQ4 and KCNQ5 (2.1 and 2.2 pS, respectively). Surprisingly, the maximal open probability (P(o)) of KCNQ3 was near unity, much higher than that of KCNQ2, KCNQ4, and KCNQ5. NEM increased the P(o) of KCNQ2, KCNQ4, and KCNQ5 by threefold to fourfold but had no effect on their unitary conductances, suggesting that the increase in macroscopic currents can be accounted for by increases in P(o). Analysis of KCNQ3/4 chimeras determined the C terminus to be responsible for the differential maximal P(o), channel expression, and NEM action between the two channels. To further localize the site of NEM action, we mutated three cysteine residues in the C terminus of KCNQ4. The C519A mutation alone ablated most of the augmentation by NEM, suggesting that NEM acts via alkylation of this residue.
Figures






Similar articles
-
Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels.Br J Pharmacol. 2002 Dec;137(8):1173-86. doi: 10.1038/sj.bjp.0704989. Br J Pharmacol. 2002. PMID: 12466226 Free PMC article.
-
Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine.J Neurosci. 2001 Aug 1;21(15):5535-45. doi: 10.1523/JNEUROSCI.21-15-05535.2001. J Neurosci. 2001. PMID: 11466425 Free PMC article.
-
Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium.J Neurosci. 2003 Jun 15;23(12):5012-9. doi: 10.1523/JNEUROSCI.23-12-05012.2003. J Neurosci. 2003. PMID: 12832524 Free PMC article.
-
M-channels: neurological diseases, neuromodulation, and drug development.Arch Neurol. 2003 Apr;60(4):496-500. doi: 10.1001/archneur.60.4.496. Arch Neurol. 2003. PMID: 12707061 Review.
-
KCNQ potassium channels: physiology, pathophysiology, and pharmacology.Pharmacol Ther. 2001 Apr;90(1):1-19. doi: 10.1016/s0163-7258(01)00116-4. Pharmacol Ther. 2001. PMID: 11448722 Review.
Cited by
-
Structure of a Ca(2+)/CaM:Kv7.4 (KCNQ4) B-helix complex provides insight into M current modulation.J Mol Biol. 2013 Jan 23;425(2):378-94. doi: 10.1016/j.jmb.2012.11.023. Epub 2012 Nov 23. J Mol Biol. 2013. PMID: 23178170 Free PMC article.
-
Inactivation as a new regulatory mechanism for neuronal Kv7 channels.Biophys J. 2007 Apr 15;92(8):2747-56. doi: 10.1529/biophysj.106.101287. Epub 2007 Jan 19. Biophys J. 2007. PMID: 17237198 Free PMC article.
-
Neuropathic pain and Kv7 voltage-gated potassium channels: The potential role of Kv7 activators in the treatment of neuropathic pain.Mol Pain. 2019 Jan-Dec;15:1744806919864256. doi: 10.1177/1744806919864256. Mol Pain. 2019. PMID: 31342847 Free PMC article. Review.
-
Redox and nitric oxide-mediated regulation of sensory neuron ion channel function.Antioxid Redox Signal. 2015 Feb 20;22(6):486-504. doi: 10.1089/ars.2014.5884. Epub 2014 Apr 15. Antioxid Redox Signal. 2015. PMID: 24735331 Free PMC article. Review.
-
β-Secretase BACE1 regulates hippocampal and reconstituted M-currents in a β-subunit-like fashion.J Neurosci. 2015 Feb 25;35(8):3298-311. doi: 10.1523/JNEUROSCI.3127-14.2015. J Neurosci. 2015. PMID: 25716831 Free PMC article.
References
-
- Axelrod D (2003) Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol 361: 1–33. - PubMed
-
- Axelrod D, Burghardt TP, Thompson NL (1984) Total internal reflection fluorescence. Annu Rev Biophys Bioeng 13: 247–268. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G (1996) K(V)LQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature 384: 78–80. - PubMed
-
- del Camino D, Holmgren M, Liu Y, Yellen G (2000) Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature 403: 321–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
