Positive and negative regulation of SMC-DNA interactions by ATP and accessory proteins
- PMID: 15175656
- PMCID: PMC449774
- DOI: 10.1038/sj.emboj.7600264
Positive and negative regulation of SMC-DNA interactions by ATP and accessory proteins
Abstract
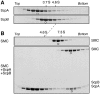
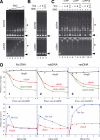
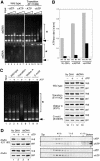
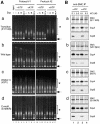
Structural maintenance of chromosomes (SMC) proteins are central regulators of higher-order chromosome dynamics from bacteria to humans. The Bacillus subtilis SMC (BsSMC) homodimer adopts a V-shaped structure with an ATP-binding catalytic domain at each end. We report here that two small proteins, ScpA and ScpB, associate with the catalytic domains of BsSMC in an ordered fashion and suppress its ATPase activity. When combined with a 'transition state' mutant of BsSMC that poorly hydrolyzes ATP, ScpA promotes stable engagement of two catalytic domains in an ATP-dependent manner. In solution, this occurs intramolecularly and closes the DNA-entry gate of an SMC dimer. ScpB further stabilizes this conformation and prevents BsSMC from binding to double-stranded DNA (dsDNA). In contrast, when the mutant BsSMC is first allowed to interact with dsDNA, subsequent addition of ScpA leads to assembly of large nucleoprotein complexes, possibly by stabilizing intermolecular engagement of the catalytic domains from different SMC dimers. We propose that the ATP-modulated engagement/disengagement cycle of SMC proteins plays both positive and negative roles in their dynamic interactions with dsDNA.
Figures

References
-
- Arumugam P, Gruber S, Tanaka K, Haering CH, Mechtler K, Nasmyth K (2003) ATP hydrolysis is required for cohesin's association with chromosomes. Curr Biol 13: 1941–1953 - PubMed
-
- Bhat MA, Philp AV, Glover DM, Bellen HJ (1996) Chromatid segregation at anaphase requires the barren product, a novel chromosome associated protein that interacts with topoisomerase II. Cell 87: 1103–1114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

