Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma
- PMID: 15175761
- PMCID: PMC2820247
- DOI: 10.1038/nature02583
Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma
Erratum in
- Nature. 2004 Aug 19;430(7002):921
Abstract
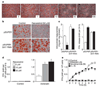
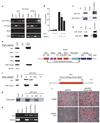
Calorie restriction extends lifespan in organisms ranging from yeast to mammals. In yeast, the SIR2 gene mediates the life-extending effects of calorie restriction. Here we show that the mammalian SIR2 orthologue, Sirt1 (sirtuin 1), activates a critical component of calorie restriction in mammals; that is, fat mobilization in white adipocytes. Upon food withdrawal Sirt1 protein binds to and represses genes controlled by the fat regulator PPAR-gamma (peroxisome proliferator-activated receptor-gamma), including genes mediating fat storage. Sirt1 represses PPAR-gamma by docking with its cofactors NCoR (nuclear receptor co-repressor) and SMRT (silencing mediator of retinoid and thyroid hormone receptors). Mobilization of fatty acids from white adipocytes upon fasting is compromised in Sirt1+/- mice. Repression of PPAR-gamma by Sirt1 is also evident in 3T3-L1 adipocytes, where overexpression of Sirt1 attenuates adipogenesis, and RNA interference of Sirt1 enhances it. In differentiated fat cells, upregulation of Sirt1 triggers lipolysis and loss of fat. As a reduction in fat is sufficient to extend murine lifespan, our results provide a possible molecular pathway connecting calorie restriction to life extension in mammals.
Figures


References
-
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. - PubMed
-
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. - PubMed
-
- Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. - PubMed
-
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, Illinois: C. C. Thomas; 1988.
-
- Lin SJ, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

