Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study
- PMID: 15176951
- PMCID: PMC1133797
- DOI: 10.1042/BJ20040036
Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study
Abstract
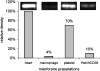
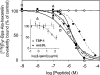
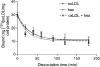
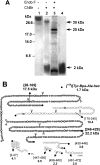
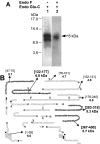
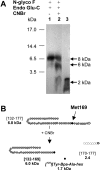
The GHRPs (growth hormone-releasing peptides) are a class of small synthetic peptides known to stimulate GH release through binding of a G-protein-coupled receptor (designated GHS-R). We have found that hexarelin, a hexapeptide member of the GHRPs, binds to another protein identified as CD36, a scavenger receptor that is expressed in various tissues, including monocytes/macrophages and the endothelial microvasculature. CD36 is involved in the endocytosis of oxLDL (oxidized low-density lipoprotein) by macrophages, and in the modulation of angiogenesis elicited by thrombospondin-1 through binding to endothelial cells. To define the binding domain for hexarelin on CD36, covalent photolabelling of CD36 followed by enzymic and chemical degradation of the photoligand-receptor complex was performed. A 8 kDa photolabelled fragment corresponding to the CD36-(Asn132-Glu177) sequence has been identified as the hexarelin-binding site. Chemical cleavage of this fragment with CNBr resulted in the release of the free ligand, suggesting that Met169 is the contact point for the ligand within the receptor binding pocket. We conclude that the binding domain for hexarelin on CD36 overlaps with that for oxLDL, which corresponds to residues Gln155-Lys183 of CD36. Hence hexarelin might interfere with the CD36-mediated uptake of modified lipoproteins by macrophages. This may contribute, at least in part, to the anti-atherosclerotic effect of GHRPs in apolipoprotein E-deficient mice.
Figures

Similar articles
-
The terminal six amino-acids of the carboxy cytoplasmic tail of CD36 contain a functional domain implicated in the binding and capture of oxidized low-density lipoprotein.Biochem J. 2002 Jun 1;364(Pt 2):507-15. doi: 10.1042/BJ20011373. Biochem J. 2002. PMID: 12023894 Free PMC article.
-
Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils.J Neurosci Res. 2009 Sep;87(12):2718-27. doi: 10.1002/jnr.22088. J Neurosci Res. 2009. PMID: 19382238
-
Structure-activity relationships of GHRP-6 azapeptide ligands of the CD36 scavenger receptor by solid-phase submonomer azapeptide synthesis.J Am Chem Soc. 2011 Aug 17;133(32):12493-506. doi: 10.1021/ja203007u. Epub 2011 Jul 21. J Am Chem Soc. 2011. PMID: 21692501
-
CD36 and macrophages in atherosclerosis.Cardiovasc Res. 2007 Aug 1;75(3):468-77. doi: 10.1016/j.cardiores.2007.03.010. Epub 2007 Mar 14. Cardiovasc Res. 2007. PMID: 17442283 Review.
-
The cardiovascular action of hexarelin.J Geriatr Cardiol. 2014 Sep;11(3):253-8. doi: 10.11909/j.issn.1671-5411.2014.03.007. J Geriatr Cardiol. 2014. PMID: 25278975 Free PMC article. Review.
Cited by
-
Therapeutic strategies to deplete macrophages in atherosclerotic plaques.Br J Clin Pharmacol. 2012 Aug;74(2):246-63. doi: 10.1111/j.1365-2125.2012.04211.x. Br J Clin Pharmacol. 2012. PMID: 22309283 Free PMC article. Review.
-
GLP-1 receptor independent pathways: emerging beneficial effects of GLP-1 breakdown products.Eat Weight Disord. 2017 Jun;22(2):231-240. doi: 10.1007/s40519-016-0352-y. Epub 2016 Dec 31. Eat Weight Disord. 2017. PMID: 28040864 Review.
-
p53 is an important regulator of CCL2 gene expression.Curr Mol Med. 2012 Sep;12(8):929-43. doi: 10.2174/156652412802480844. Curr Mol Med. 2012. PMID: 22804246 Free PMC article.
-
Atherosclerosis treatment with nanoagent: potential targets, stimulus signals and drug delivery mechanisms.Front Bioeng Biotechnol. 2023 Jun 19;11:1205751. doi: 10.3389/fbioe.2023.1205751. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37404681 Free PMC article. Review.
-
Development and Characterization of an 18F-labeled Ghrelin Peptidomimetic for Imaging the Cardiac Growth Hormone Secretagogue Receptor.Mol Imaging. 2018 Jan-Dec;17:1536012118809587. doi: 10.1177/1536012118809587. Mol Imaging. 2018. PMID: 30394854 Free PMC article.
References
-
- Howard A. D., Feighner S. D., Cully D. F., Arena J. P., Liberator P. A., Rosenblum C. I., Hamelin M., Hreniuk D. L., Palyha O. C., Anderson J., et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. - PubMed
-
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature (London) 1999;402:656–660. - PubMed
-
- Muccioli G., Broglio F., Valetto M. R., Ghe C., Catapano F., Graziani A., Papotti M., Bisi G., Deghenghi R., Ghigo E. Growth hormone-releasing peptides and the cardiovascular system. Ann. Endocrinol. (Paris) 2000;61:27–31. - PubMed
-
- Ghigo E., Arvat E., Giordano R., Broglio F., Gianotti L., Maccario M., Bisi G., Graziani A., Papotti M., Muccioli G., et al. Biologic activities of growth hormone secretagogues in humans. Endocrine. 2001;14:87–93. - PubMed
-
- Papotti M., Ghe C., Cassoni P., Catapano F., Deghenghi R., Ghigo D., Muccioli G. Growth hormone secretagogue binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 2000;85:3803–3807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

