The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation
- PMID: 15176985
- PMCID: PMC436058
- DOI: 10.1186/1471-2199-5-3
The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation
Abstract
Background: Exposure of Leishmania promastigotes to the temperature of their mammalian hosts results in the induction of a typical heat shock response. It has been suggested that heat shock proteins play an important role in parasite survival and differentiation.
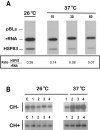
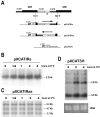
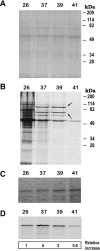
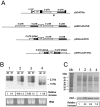
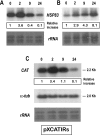
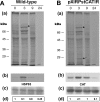
Results: Here we report the studies on the expression of the heat shock protein 83 (HSP83) genes of Leishmania infantum. Confirming previous observations for other Leishmania species, we found that the L. infantum HSP83 transcripts also show a temperature-dependent accumulation that is controlled by a post-transcriptional mechanism involving sequences located in the 3'-untranslated region (3'-UTR). However, contrary to that described for L. amazonensis, the accumulation of the HSP83 transcripts in L. infantum is dependent on active protein synthesis. The translation of HSP83 transcripts is enhanced during heat shock and, as first described in L. amazonensis, we show that the 3'-UTR of the L. infantum HSP83 gene is essential for this translational control. Measurement of the steady-state levels of HSP83 transcripts along the promastigote-to-amastigote differentiation evidenced a specific profile of HSP83 RNAs: after an initial accumulation of HSP83 transcripts observed short after (2 h) incubation in the differentiation conditions, the amount of HSP83 RNA decreased to a steady-state level lower than in undifferentiated promastigotes. We show that this transient accumulation is linked to the presence of the 3'-UTR and flanking regions. Again, an 8-fold increase in translation of the HSP83 transcripts is observed short after the initiation of the axenic differentiation, but it is not sustained after 9 h.
Conclusions: This transient expression of HSP83 genes could be relevant for the differentiation of Leishmania, and the underlying regulatory mechanism may be part of the developmental program of this parasite.
Figures

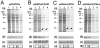

Similar articles
-
Developmental regulation of heat shock protein 83 in Leishmania. 3' processing and mRNA stability control transcript abundance, and translation id directed by a determinant in the 3'-untranslated region.J Biol Chem. 2001 Dec 21;276(51):47922-9. doi: 10.1074/jbc.M108271200. Epub 2001 Oct 11. J Biol Chem. 2001. PMID: 11598129
-
Cell-cycle-dependent translation of histone mRNAs is the key control point for regulation of histone biosynthesis in Leishmania infantum.Biochem J. 2004 May 1;379(Pt 3):617-25. doi: 10.1042/BJ20031522. Biochem J. 2004. PMID: 14766017 Free PMC article.
-
Characterization and developmental gene regulation of a large gene family encoding amastin surface proteins in Leishmania spp.Mol Biochem Parasitol. 2005 Apr;140(2):205-20. doi: 10.1016/j.molbiopara.2005.01.006. Mol Biochem Parasitol. 2005. PMID: 15760660
-
Global gene expression in Leishmania.Int J Parasitol. 2007 Aug;37(10):1077-86. doi: 10.1016/j.ijpara.2007.04.011. Epub 2007 May 6. Int J Parasitol. 2007. PMID: 17574557 Review.
-
The power of the 3' UTR: translational control and development.Nat Rev Genet. 2003 Aug;4(8):626-37. doi: 10.1038/nrg1125. Nat Rev Genet. 2003. PMID: 12897774 Review.
Cited by
-
Coordinate regulation of a family of promastigote-enriched mRNAs by the 3'UTR PRE element in Leishmania mexicana.Mol Biochem Parasitol. 2008 Jan;157(1):54-64. doi: 10.1016/j.molbiopara.2007.10.001. Epub 2007 Oct 5. Mol Biochem Parasitol. 2008. PMID: 18023890 Free PMC article.
-
Regulation of Leishmania (L.) amazonensis protein expression by host T cell dependent responses: differential expression of oligopeptidase B, tryparedoxin peroxidase and HSP70 isoforms in amastigotes isolated from BALB/c and BALB/c nude mice.PLoS Negl Trop Dis. 2015 Feb 18;9(2):e0003411. doi: 10.1371/journal.pntd.0003411. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25692783 Free PMC article.
-
How do trypanosomes change gene expression in response to the environment?Protoplasma. 2012 Apr;249(2):223-38. doi: 10.1007/s00709-011-0282-5. Epub 2011 May 20. Protoplasma. 2012. PMID: 21594757 Free PMC article. Review.
-
Heat Shock Proteins as the Druggable Targets in Leishmaniasis: Promises and Perils.Infect Immun. 2021 Jan 19;89(2):e00559-20. doi: 10.1128/IAI.00559-20. Print 2021 Jan 19. Infect Immun. 2021. PMID: 33139381 Free PMC article. Review.
-
Key role of the 3' untranslated region in the cell cycle regulated expression of the Leishmania infantum histone H2A genes: minor synergistic effect of the 5' untranslated region.BMC Mol Biol. 2009 May 21;10:48. doi: 10.1186/1471-2199-10-48. BMC Mol Biol. 2009. PMID: 19460148 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

