Therapeutic and diagnostic implications of Hsp90 activation
- PMID: 15177193
- PMCID: PMC7128344
- DOI: 10.1016/j.molmed.2004.04.006
Therapeutic and diagnostic implications of Hsp90 activation
Abstract
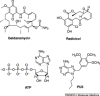
The molecular chaperone heat-shock protein 90 (Hsp90) is involved in the stabilization and conformational maturation of many signaling proteins that are deregulated in cancers. Hsp90 inhibition results in the proteasomal degradation of these client proteins and leads to potent antitumor activity. The Hsp90 inhibitor 17-allylaminogeldanamycin (17-AAG) is presently in clinical trials. Recent work has identified the role of Hsp90 in multiple signal transduction pathways and revealed that the molecular mechanism of tumor selectivity by Hsp90 inhibitors is the result of an activated, high-affinity conformation of Hsp90 in tumors. This review discusses these recent advances in the understanding of tumor Hsp90 for the treatment and diagnosis of cancer. In addition, the role of Hsp90 in non-oncological diseases will also be discussed.
Figures



References
-
- Isaacs J.S. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. - PubMed
-
- Pratt W.B, Toft D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 2003;228:111–133. - PubMed
-
- Schulte T.W, Neckers L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998;42:273–279. - PubMed
-
- Xu W. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 2001;276:3702–3708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

