A cardiac arrhythmia syndrome caused by loss of ankyrin-B function
- PMID: 15178757
- PMCID: PMC428486
- DOI: 10.1073/pnas.0402546101
A cardiac arrhythmia syndrome caused by loss of ankyrin-B function
Abstract
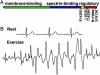
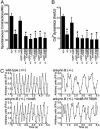
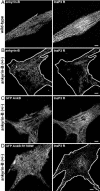
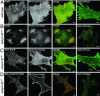
220-kDa ankyrin-B is required for coordinated assembly of Na/Ca exchanger, Na/K ATPase, and inositol trisphosphate (InsP(3)) receptor at transverse-tubule/sarcoplasmic reticulum sites in cardiomyocytes. A loss-of-function mutation of ankyrin-B identified in an extended kindred causes a dominantly inherited cardiac arrhythmia, initially described as type 4 long QT syndrome. Here we report the identification of eight unrelated probands harboring ankyrin-B loss-of-function mutations, including four previously undescribed mutations, whose clinical features distinguish the cardiac phenotype associated with loss of ankyrin-B activity from classic long QT syndromes. Humans with ankyrin-B mutations display varying degrees of cardiac dysfunction including bradycardia, sinus arrhythmia, idiopathic ventricular fibrillation, catecholaminergic polymorphic ventricular tachycardia, and risk of sudden death. However, a prolonged rate-corrected QT interval was not a consistent feature, indicating that ankyrin-B dysfunction represents a clinical entity distinct from classic long QT syndromes. The mutations are localized in the ankyrin-B regulatory domain, which distinguishes function of ankyrin-B from ankyrin-G in cardiomyocytes. All mutations abolish ability of ankyrin-B to restore abnormal Ca(2+) dynamics and abnormal localization and expression of Na/Ca exchanger, Na/K ATPase, and InsP(3)R in ankyrin-B(+/-) cardiomyocytes. This study, considered together with the first description of ankyrin-B mutation associated with cardiac dysfunction, supports a previously undescribed paradigm for human disease due to abnormal coordination of multiple functionally related ion channels and transporters, in this case the Na/K ATPase, Na/Ca exchanger, and InsP(3) receptor.
Figures

Similar articles
-
Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death.Nature. 2003 Feb 6;421(6923):634-9. doi: 10.1038/nature01335. Nature. 2003. PMID: 12571597
-
Defining new insight into atypical arrhythmia: a computational model of ankyrin-B syndrome.Am J Physiol Heart Circ Physiol. 2010 Nov;299(5):H1505-14. doi: 10.1152/ajpheart.00503.2010. Epub 2010 Aug 20. Am J Physiol Heart Circ Physiol. 2010. PMID: 20729400 Free PMC article.
-
Novel Variant in the ANK2 Membrane-Binding Domain Is Associated With Ankyrin-B Syndrome and Structural Heart Disease in a First Nations Population With a High Rate of Long QT Syndrome.Circ Cardiovasc Genet. 2017 Jan;10(1):e001537. doi: 10.1161/CIRCGENETICS.116.001537. Circ Cardiovasc Genet. 2017. PMID: 28196901 Free PMC article.
-
Ankyrin-based cardiac arrhythmias: a new class of channelopathies due to loss of cellular targeting.Curr Opin Cardiol. 2005 May;20(3):189-93. doi: 10.1097/01.hco.0000160372.95116.3e. Curr Opin Cardiol. 2005. PMID: 15861006 Review.
-
Cardiac ankyrins: Essential components for development and maintenance of excitable membrane domains in heart.Cardiovasc Res. 2006 Jul 1;71(1):22-9. doi: 10.1016/j.cardiores.2006.03.018. Epub 2006 Mar 28. Cardiovasc Res. 2006. PMID: 16650839 Review.
Cited by
-
Identification and characterization of two ankyrin-B isoforms in mammalian heart.Cardiovasc Res. 2015 Sep 1;107(4):466-77. doi: 10.1093/cvr/cvv184. Epub 2015 Jun 24. Cardiovasc Res. 2015. PMID: 26109584 Free PMC article.
-
Gene expression analyses implicate an alternative splicing program in regulating contractile gene expression and serum response factor activity in mice.PLoS One. 2013;8(2):e56590. doi: 10.1371/journal.pone.0056590. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437181 Free PMC article.
-
A single divergent exon inhibits ankyrin-B association with the plasma membrane.J Biol Chem. 2013 May 24;288(21):14769-79. doi: 10.1074/jbc.M113.465328. Epub 2013 Apr 8. J Biol Chem. 2013. PMID: 23569209 Free PMC article.
-
LQTS gene LOVD database.Hum Mutat. 2010 Nov;31(11):E1801-10. doi: 10.1002/humu.21341. Hum Mutat. 2010. PMID: 20809527 Free PMC article.
-
Sudden arrhythmic death syndrome: a national survey of sudden unexplained cardiac death.Heart. 2007 May;93(5):601-5. doi: 10.1136/hrt.2006.099598. Epub 2007 Jan 19. Heart. 2007. PMID: 17237131 Free PMC article.
References
-
- Towbin, J. A. & Vatta, M. (2001) Am. J. Med. 110, 385-398. - PubMed
-
- Splawski, I., Shen, J., Timothy, K. W., Lehmann, M. H., Priori, S., Robinson, J. L., Moss, A. J., Schwartz, P. J., Towbin, J. A., Vincent, G. M. & Keating, M. T. (2000) Circulation 102, 1178-1185. - PubMed
-
- Priori, S. G., Napolitano, C. & Vicentini, A. (2003) J. Interv. Cardiol. Electrophysiol. 9, 93-101. - PubMed
-
- Moss, A. J. (2003) J. Am. Med. Assoc. 289, 2041-2044. - PubMed
-
- Mohler, P. J., Schott, J. J., Gramolini, A. O., Dilly, K. W., Guatimosim, S., duBell, W. H., Song, L. S., Haurogne, K., Kyndt, F., Ali, M. E., et al. (2003) Nature 421, 634-639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

