Structure of vaccinia complement protein in complex with heparin and potential implications for complement regulation
- PMID: 15178763
- PMCID: PMC428448
- DOI: 10.1073/pnas.0400744101
Structure of vaccinia complement protein in complex with heparin and potential implications for complement regulation
Retraction in
-
Retraction for Ganesh et al., Structure of vaccinia complement protein in complex with heparin and potential implications for complement regulation.Proc Natl Acad Sci U S A. 2018 Jul 17;115(29):E6965. doi: 10.1073/pnas.1806429115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987022 Free PMC article. No abstract available.
Expression of concern in
-
Editorial Expression of Concern for multiple articles.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6551. doi: 10.1073/pnas.1003210107. Proc Natl Acad Sci U S A. 2010. PMID: 28277629 Free PMC article. No abstract available.
Abstract
Vaccinia virus complement control protein (VCP), a homolog of the regulators of the complement activation family of proteins, inhibits complement activation through mechanisms similar to human fluid-phase complement regulators factor H and C4b-binding protein. VCP has a heparin-binding activity that assists vaccinia in host interactions. Interaction with cell-surface polyanions like heparin is centrally important in the functioning of fluid-phase complement regulators and is the basis of host-target discrimination by the alternative pathway. We report the structure of VCP in complex with a heparin decasaccharide, which reveals changes in VCP that might be pertinent to complement regulation. Properties that VCP shares with fluid-phase complement regulators suggest that such conformational changes may be of relevance in the functioning of other complement regulators. Additionally, comparison of VCP-heparin interactions with potentially similar interactions in factor H might enable understanding of the structural basis of familial hemolytic uremic syndrome, attributed to mutational disruption of heparin and C3b binding by factor H.
Figures
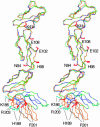
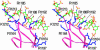
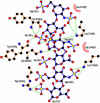
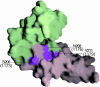

Comment in
-
Findings of Research Misconduct.Fed Regist. 2018 Apr 16;83(73):16370-16371. Fed Regist. 2018. PMID: 30407470 Free PMC article. No abstract available.
References
-
- Kotwal, G. J. & Moss, B. (1988) Nature 335, 176–178. - PubMed
-
- Kotwal, G. J. (2000) Immunol. Today 21, 243–248. - PubMed
-
- Kotwal, G. J., Issacs, S. T., McKenzie, R., Frank, M. M. & Moss, B. (1990) Science 250, 827–830. - PubMed
-
- Rosengard, A. M., Alnonso, L. C., Korb, L. C., Baldwin, W. M., Sanfilippo, F., Turka, L. A. & Ahearn, J. M. (1999) Mol. Immunol. 36, 685–697. - PubMed
-
- Smith, S. A., Sreenivasan, R., Krishnasamy, G., Judge, K. W., Murthy, K. H., Arjunwadkar, S. J., Pugh, D. R. & Kotwal, G. J. (2003) Biochim. Biophys. Acta 1650, 30–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

