The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization
- PMID: 15181200
- PMCID: PMC428480
- DOI: 10.1073/pnas.0402959101
The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization
Abstract
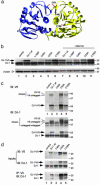
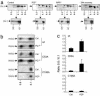
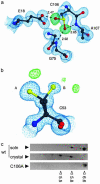
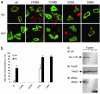
Loss-of-function DJ-1 mutations can cause early-onset Parkinson's disease. The function of DJ-1 is unknown, but an acidic isoform accumulates after oxidative stress, leading to the suggestion that DJ-1 is protective under these conditions. We addressed whether this represents a posttranslational modification at cysteine residues by systematically mutating cysteine residues in human DJ-1. WT or C53A DJ-1 was readily oxidized in cultured cells, generating a pI 5.8 isoform, but an artificial C106A mutant was not. We observed a cysteine-sulfinic acid at C106 in crystalline DJ-1 but no modification of C53 or C46. Oxidation of DJ-1 was promoted by the crystallization procedure. In addition, oxidation-induced mitochondrial relocalization of DJ-1 and protection against cell death were abrogated in C106A but not C53A or C46A. We suggest that DJ-1 protects against neuronal death, and that this is signaled by acidification of the key cysteine residue, C106.
Figures

References
-
- Dawson, T. M. & Dawson, V. L. (2003) Science 302, 819-822. - PubMed
-
- Petrucelli, L., O'Farrell, C., Lockhart, P. J., Baptista, M., Kehoe, K., Vink, L., Choi, P., Wolozin, B., Farrer, M., Hardy, J., et al. (2002) Neuron 36, 1007-1019. - PubMed
-
- Darios, F., Corti, O., Lucking, C. B., Hampe, C., Muriel, M. P., Abbas, N., Gu, W. J., Hirsch, E. C., Rooney, T., Ruberg, M., et al. (2003) Hum. Mol. Genet. 12, 517-526. - PubMed
-
- Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., Dekker, M. C., Squitieri, F., Ibanez, P., Joosse, M., et al. (2003) Science 299, 256-259. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

