Characterization and heterologous gene expression of a novel esterase from Lactobacillus casei CL96
- PMID: 15184114
- PMCID: PMC427766
- DOI: 10.1128/AEM.70.6.3213-3221.2004
Characterization and heterologous gene expression of a novel esterase from Lactobacillus casei CL96
Abstract
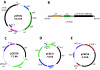
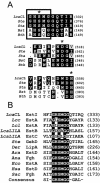
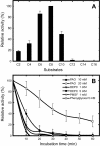
A novel esterase gene (estI) of Lactobacillus casei CL96 was localized on a 3.3-kb BamHI DNA fragment containing an open reading frame (ORF) of 1,800 bp. The ORF of estI was isolated by PCR and expressed in Escherichia coli, the methylotrophic bacterium Methylobacterium extorquens, and the methylotrophic yeast Pichia pastoris under the control of T7, methanol dehydrogenase (P(mxaF)), and alcohol oxidase (AOX1) promoters, respectively. The amino acid sequence of EstI indicated that the esterase is a novel member of the GHSMG family of lipolytic enzymes and that the enzyme contains a lipase-like catalytic triad, consisting of Ser325, Asp516, and His558. E. coli BL21(DE3)/pLysS containing estI expressed a novel 67.5-kDa protein corresponding to EstI in an N-terminal fusion with the S. tag peptide. The recombinant L. casei CL96 EstI protein was purified to electrophoretic homogeneity in a one-step affinity chromatography procedure on S-protein agarose. The optimum pH and temperature of the purified enzyme were 7.0 and 37 degrees C, respectively. Among the pNP (p-nitrophenyl) esters tested, the most selective substrate was pNP-caprylate (C(8)), with K(m) and k(cat) values of 14 +/- 1.08 microM and 1,245 +/- 42.3 S(-1), respectively.
Figures




Similar articles
-
Intracellular esterase from Lactobacillus casei LILA: nucleotide sequencing, purification, and characterization.J Dairy Sci. 2003 Apr;86(4):1118-29. doi: 10.3168/jds.s0022-0302(03)73694-7. J Dairy Sci. 2003. PMID: 12741535
-
Functional expression of a novel methanol-stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent-free system.BMC Biotechnol. 2020 Jun 29;20(1):36. doi: 10.1186/s12896-020-00622-1. BMC Biotechnol. 2020. PMID: 32600313 Free PMC article.
-
Cloning and characterization of an intracellular esterase from the wine-associated lactic acid bacterium Oenococcus oeni.Appl Environ Microbiol. 2009 Nov;75(21):6729-35. doi: 10.1128/AEM.01563-09. Epub 2009 Sep 4. Appl Environ Microbiol. 2009. PMID: 19734337 Free PMC article.
-
Cloning, Expression and Characterization of a Thermostable Esterase HydS14 from Actinomadura sp. Strain S14 in Pichia pastoris.Int J Mol Sci. 2015 Jun 12;16(6):13579-94. doi: 10.3390/ijms160613579. Int J Mol Sci. 2015. PMID: 26075873 Free PMC article.
-
Cloning, overexpression, and properties of a new thermophilic and thermostable esterase with sequence similarity to hormone-sensitive lipase subfamily from the archaeon Archaeoglobus fulgidus.Arch Biochem Biophys. 2000 Jan 1;373(1):182-92. doi: 10.1006/abbi.1999.1497. Arch Biochem Biophys. 2000. PMID: 10620337
Cited by
-
Novel, versatile, and tightly regulated expression system for Escherichia coli strains.Appl Environ Microbiol. 2010 Aug;76(15):5058-66. doi: 10.1128/AEM.00413-10. Epub 2010 Jun 18. Appl Environ Microbiol. 2010. PMID: 20562288 Free PMC article.
-
Improved method for qualitative screening of lipolytic bacterial strains.MethodsX. 2018 Feb 1;5:68-74. doi: 10.1016/j.mex.2018.01.004. eCollection 2018. MethodsX. 2018. PMID: 30622910 Free PMC article.
-
Multicopy integration and expression of heterologous genes in Methylobacterium extorquens ATCC 55366.Appl Environ Microbiol. 2006 Jan;72(1):753-9. doi: 10.1128/AEM.72.1.753-759.2006. Appl Environ Microbiol. 2006. PMID: 16391115 Free PMC article.
-
Heterologous expression of leader-less pga gene in Pichia pastoris: intracellular production of prokaryotic enzyme.BMC Biotechnol. 2010 Feb 3;10:7. doi: 10.1186/1472-6750-10-7. BMC Biotechnol. 2010. PMID: 20128906 Free PMC article.
-
Molecular cloning and characterization of a newly isolated pyrethroid-degrading esterase gene from a genomic library of Ochrobactrum anthropi YZ-1.PLoS One. 2013 Oct 14;8(10):e77329. doi: 10.1371/journal.pone.0077329. eCollection 2013. PLoS One. 2013. PMID: 24155944 Free PMC article.
References
-
- Alvarez, M. E., M. V. Augier, and J. Baratti. 1999. Characterization of a thermostable esterase activity from the moderate thermophile Bacillus licheniformis. Biosci. Biotechnol. Biochem. 63:1865-1870. - PubMed
-
- Bélanger, E. 1998. Genetic identification of Lactobacillus and Bifidobacteria species. M.Sc. thesis. McGill University, Montreal, Canada.
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, N. Gregor, G. F. Davis, H. A. Kirkpatrick, M. A. Goeden, J. D. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Bourque, D., Y. Pomerleau, and D. Groleau. 1995. High-cell-density production of poly-β-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: production of high-molecular-mass PHB. Appl. Microbiol. Biotechnol. 44:367-376.
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

