In vivo production of artificial nonribosomal peptide products in the heterologous host Escherichia coli
- PMID: 15184122
- PMCID: PMC427719
- DOI: 10.1128/AEM.70.6.3282-3291.2004
In vivo production of artificial nonribosomal peptide products in the heterologous host Escherichia coli
Abstract
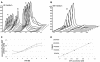
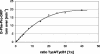
Nonribosomal peptide synthetases represent the enzymatic assembly lines for the biosynthesis of pharmacologically relevant natural peptides, e.g., cyclosporine, vancomycin, and penicillin. Due to their modular organization, in which every module accounts for the incorporation of a single amino acid, artificial assembly lines for the production of novel peptides can be constructed by biocombinatorial approaches. Once transferred into an appropriate host, these hybrid synthetases could facilitate the bioproduction of basically any peptide-based molecule. In the present study, we describe the fermentative production of the cyclic dipeptide D-Phe-Pro-diketopiperazine, as a prototype for the exploitation of the heterologous host Escherichia coli, and the use of artificial nonribosomal peptide synthetases. E. coli provides a tremendous potential for genetic engineering and was manipulated in our study by stable chromosomal integration of the 4'-phosphopantetheine transferase gene sfp to ensure heterologous production of fully active holoenzmyes. D-Phe-Pro-diketopiperazine is formed by the TycA/TycB1 system, whose components represent the first two modules for tyrocidine biosynthesis in Bacillus brevis. Coexpression of the corresponding genes in E. coli gave rise to the production of the expected diketopiperazine product, demonstrating the functional interaction of both modules in the heterologous environment. Furthermore, the cyclic dipeptide is stable and not toxic to E. coli and is secreted into the culture medium without the need for any additional factors. Parameters affecting the productivity were comprehensively investigated, including various genetic setups, as well as variation of medium composition and temperature. By these means, the overall productivity of the artificial system could be enhanced by over 400% to yield about 9 mg of D-Phe-Pro-diketopiperazine/liter. As a general tool, this approach could allow the sustainable bioproduction of peptides, e.g., those used as pharmaceuticals or fine chemicals.
Figures



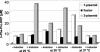
Similar articles
-
Recognition of hybrid peptidyl carrier proteins/acyl carrier proteins in nonribosomal peptide synthetase modules by the 4'-phosphopantetheinyl transferases AcpS and Sfp.J Biol Chem. 2002 May 10;277(19):17023-31. doi: 10.1074/jbc.M200120200. Epub 2002 Feb 26. J Biol Chem. 2002. PMID: 11867633
-
Selective interaction between nonribosomal peptide synthetases is facilitated by short communication-mediating domains.Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15585-90. doi: 10.1073/pnas.0404932101. Epub 2004 Oct 21. Proc Natl Acad Sci U S A. 2004. PMID: 15498872 Free PMC article.
-
Portability of epimerization domain and role of peptidyl carrier protein on epimerization activity in nonribosomal peptide synthetases.Biochemistry. 2001 Dec 25;40(51):15824-34. doi: 10.1021/bi011595t. Biochemistry. 2001. PMID: 11747460
-
Robust platform for de novo production of heterologous polyketides and nonribosomal peptides in Escherichia coli.Org Biomol Chem. 2007 Feb 21;5(4):593-602. doi: 10.1039/b615589h. Epub 2006 Dec 21. Org Biomol Chem. 2007. PMID: 17285165 Review.
-
Combinatorial biosynthesis of non-ribosomal peptides.Comb Chem High Throughput Screen. 2003 Sep;6(6):527-40. doi: 10.2174/138620703106298707. Comb Chem High Throughput Screen. 2003. PMID: 14529378 Review.
Cited by
-
Efficient nonenzymatic cyclization and domain shuffling drive pyrrolopyrazine diversity from truncated variants of a fungal NRPS.Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25614-25623. doi: 10.1073/pnas.1913080116. Epub 2019 Dec 4. Proc Natl Acad Sci U S A. 2019. PMID: 31801877 Free PMC article.
-
Cell-free synthetic biology for in vitro biosynthesis of pharmaceutical natural products.Synth Syst Biotechnol. 2018 Feb 17;3(2):83-89. doi: 10.1016/j.synbio.2018.02.002. eCollection 2018 Jun. Synth Syst Biotechnol. 2018. PMID: 29900420 Free PMC article. Review.
-
Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds.Mar Drugs. 2014 Jun 10;12(6):3516-59. doi: 10.3390/md12063516. Mar Drugs. 2014. PMID: 24918453 Free PMC article. Review.
-
Efficient rational modification of non-ribosomal peptides by adenylation domain substitution.Nat Commun. 2020 Sep 11;11(1):4554. doi: 10.1038/s41467-020-18365-0. Nat Commun. 2020. PMID: 32917865 Free PMC article.
-
Metabolic engineering for the production of natural products.Annu Rev Chem Biomol Eng. 2011;2:211-36. doi: 10.1146/annurev-chembioeng-061010-114209. Annu Rev Chem Biomol Eng. 2011. PMID: 22432617 Free PMC article. Review.
References
-
- Bartolome, B., Y. Jubete, E. Martinez, and F. de la Cruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. - PubMed
-
- Belshaw, P. J., C. T. Walsh, and T. Stachelhaus. 1999. Aminoacyl-CoAs as probes of condensation domain selectivity in nonribosomal peptide synthesis. Science 284:486-489. - PubMed
-
- Bergendahl, V., U. Linne, and M. A. Marahiel. 2002. Mutational analysis of the C-domain in nonribosomal peptide synthesis. Eur. J. Biochem. 269:620-629. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Brady, S. F., C. J. Chao, J. Handelsman, and J. Clardy. 2001. Cloning and heterologous expression of a natural product biosynthetic gene cluster from eDNA. Org. Lett. 3:1981-1984. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

