Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1
- PMID: 15184403
- PMCID: PMC2172370
- DOI: 10.1083/jcb.200312172
Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1
Abstract
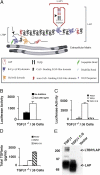
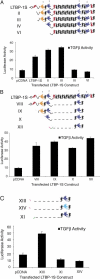
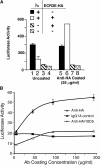
Transforming growth factor-betas (TGF-beta) are secreted as inactive complexes containing the TGF-beta, the TGF-beta propeptide, also called the latency-associated protein (LAP), and the latent TGF-beta binding protein (LTBP). Extracellular activation of this complex is a critical but incompletely understood step in TGF-beta regulation. We have investigated the role of LTBP in modulating TGF-beta generation by the integrin alphaVbeta6. We show that even though alphavbeta6 recognizes an RGD on LAP, LTBP-1 is required for alphaVbeta6-mediated latent TGF-beta activation. The domains of LTBP-1 necessary for activation include the TGF-beta propeptide-binding domain and a basic amino acid sequence (hinge domain) with ECM targeting properties. Our results demonstrate an LTBP-1 isoform-specific function in alphaVbeta6-mediated latent TGF-beta activation; LTBP-3 is unable to substitute for LTBP-1 in this assay. The results reveal a functional role for LTBP-1 in latent TGF-beta activation and suggest that activation of specific latent complexes is regulated by distinct mechanisms that may be determined by the LTBP isoform and its potential interaction with the matrix.
Copyright the Rockefeller University Press
Figures

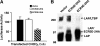


References
-
- Abe, M., J.G. Harpel, C.N. Metz, I. Nunes, D.J. Loskutoff, and D.B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276–284. - PubMed
-
- Abe, M., N. Oda, and Y. Sato. 1998. Cell-associated activation of latent transforming growth factor-beta by calpain. J. Cell. Physiol. 174:186–193. - PubMed
-
- Annes, J.P., J.S. Munger, and D.B. Rifkin. 2003. Making sense of latent TGFbeta activation. J. Cell Sci. 116:217–224. - PubMed
-
- Annes, J.P., D.B. Rifkin, and J.S. Munger. 2002. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 511:65–68. - PubMed
-
- Blobe, G.C., W.P. Schiemann, and H.F. Lodish. 2000. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342:1350–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

