Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1-->3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders
- PMID: 15184460
- PMCID: PMC427860
- DOI: 10.1128/JCM.42.6.2733-2741.2004
Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1-->3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders
Abstract
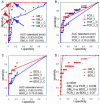
The establishment of an optimal noninvasive method for diagnosing invasive aspergillosis (IA) is needed to improve the management of this life-threatening infection in patients with hematological disorders, and a number of noninvasive tests for IA that target different fungal components, including galactomannan, (1-->3)-beta-d-glucan (BDG), and Aspergillus DNA, have been developed. In this study, we prospectively evaluated the diagnostic potential of three noninvasive tests for IA that were used in a weekly screening strategy: the double-sandwich enzyme-linked immunosorbent assay (ELISA) for galactomannan (Platelia Aspergillus), a real-time PCR assay for Aspergillus DNA (GeniQ-Asper), and an assay for BDG (beta-glucan Wako). We analyzed 149 consecutive treatment episodes in 96 patients with hematological disorders who were at high risk for IA and diagnosed 9 proven IA cases, 2 probable IA cases, and 13 possible invasive fugal infections. In a receiver-operating characteristic (ROC) analysis, the area under the ROC curve was greatest for ELISA, using two consecutive positive results (0.97; P = 0.036 for ELISA versus PCR, P = 0.055 for ELISA versus BDG). Based on the ROC curve, the cutoff for the ELISA could be reduced to an optical density index (O.D.I.) of 0.6. With the use of this cutoff for ELISA and cutoffs for PCR and BDG that give a comparable level of specificity, the sensitivity/specificity/positive predictive value/negative predictive value of the ELISA and the PCR and BDG tests were 1.00/0.93/0.55/1.00, 0.55/0.93/0.40/0.96, and 0.55/0.93/0.40/0.96, respectively. In conclusion, among these weekly screening tests for IA, the double-sandwich ELISA test was the most sensitive at predicting the diagnosis of IA in high-risk patients with hematological disorders, using a reduced cutoff of 0.6 O.D.I.
Figures


References
-
- Andreas, S., S. Heindl, C. Wattky, K. Moller, and R. Ruchel. 2000. Diagnosis of pulmonary aspergillosis using optical brighteners. Eur. Respir. J. 15:407-411. - PubMed
-
- Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. - PubMed
-
- Bowden, R., P. Chandrasekar, M. H. White, X. Li, L. Pietrelli, M. Gurwith, J. A. van Burik, M. Laverdiere, S. Safrin, and J. R. Wingard. 2002. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35:359-366. - PubMed
-
- Bretagne, S., J. M. Costa, E. Bart-Delabesse, N. Dhedin, C. Rieux, and C. Cordonnier. 1998. Comparison of serum galactomannan antigen detection and competitive polymerase chain reaction for diagnosing invasive aspergillosis. Clin. Infect. Dis. 26:1407-1412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

