Virosomes as new carrier system for cancer vaccines
- PMID: 15185010
- PMCID: PMC11032935
- DOI: 10.1007/s00262-004-0545-5
Virosomes as new carrier system for cancer vaccines
Abstract
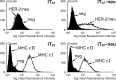
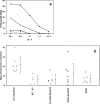
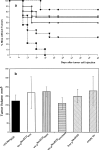
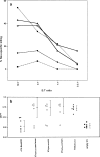
HER-2/neu, a tumor-associated antigen (TAAg), plays a critical role in oncogenesis of various tumor types, and its selective overexpression by malignant tumor cells makes it an ideal target for immunotherapy. A prerequisite for clinical vaccines is the construction of safe and highly immunogenic reagents able to generate efficient immune responses against TAAg. Previous protein vaccines, consisting of the extracellular domain of HER-2/neu (pNeuECD), were shown to elicit an immune response that did not provide protection from transplantable tumors expressing HER-2/neu. Here we showed that virosomes, which consist of reconstituted viral envelopes without viral genetic material, can act as a carrier and an adjuvant for a truncated protein pNeuECD. Mice vaccinated with pNeuECD either encapsulated in virosomes or bound to the virosomal membrane (Vir-pNeuECD), generated rNeu-specific humoral and cytotoxic immune responses. In addition, Vir-p(NeuECD) induced significant tumor rejection and additionally did not lead to delayed tumor formation when compared with free pNeuECD in complete Freund's adjuvant. There was no difference between the virosomal constructs. Taken together these results suggest that virosomes, as clinically approved safe vaccines, can be used to elicit both humoral and cell-mediated responses against TAAg and induce tumor rejection. Our model is providing important preclinical data to design human vaccination trials for patients with tumors overexpressing HER-2/neu, either as a primary vaccination or as a boost in combination with other vaccines in a context of an adjuvant treatment plan.
Figures

Similar articles
-
Plasmid-based vaccines encoding rat neu and immune stimulatory molecules can elicit rat neu-specific immunity.Mol Cancer Ther. 2003 Oct;2(10):995-1002. Mol Cancer Ther. 2003. PMID: 14578464
-
Targeting her-2/neu with antirat Neu virosomes for cancer therapy.Cancer Res. 2002 Jan 15;62(2):437-44. Cancer Res. 2002. PMID: 11809693
-
A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study.Breast Cancer Res Treat. 2010 Feb;119(3):673-83. doi: 10.1007/s10549-009-0666-9. Breast Cancer Res Treat. 2010. PMID: 20092022 Clinical Trial.
-
The virosome concept for influenza vaccines.Vaccine. 2005 Jul 8;23 Suppl 1:S26-38. doi: 10.1016/j.vaccine.2005.04.026. Vaccine. 2005. PMID: 16026906 Review.
-
Cellular immunity to the Her-2/neu protooncogene.Adv Cancer Res. 2002;85:101-44. doi: 10.1016/s0065-230x(02)85004-7. Adv Cancer Res. 2002. PMID: 12374283 Review.
Cited by
-
Pseudovirions as vehicles for the delivery of siRNA.Pharm Res. 2010 Mar;27(3):400-20. doi: 10.1007/s11095-009-0012-2. Epub 2009 Dec 9. Pharm Res. 2010. PMID: 19998056 Free PMC article.
-
Nanovaccines for cancer immunotherapy.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019 Sep;11(5):e1559. doi: 10.1002/wnan.1559. Epub 2019 Jun 6. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019. PMID: 31172659 Free PMC article. Review.
-
Cancer Drug Targeting: Molecular Mechanism, Approaches, and Regulatory Framework.Curr Pharm Des. 2025;31(22):1767-1780. doi: 10.2174/0113816128364722250126172914. Curr Pharm Des. 2025. PMID: 39957701 Review.
-
Natural carrier systems in cancer vaccines and immunotherapy.Hum Vaccin Immunother. 2025 Dec;21(1):2535787. doi: 10.1080/21645515.2025.2535787. Epub 2025 Jul 24. Hum Vaccin Immunother. 2025. PMID: 40707012 Free PMC article. Review.
-
Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine.Clin Dev Immunol. 2013;2013:387023. doi: 10.1155/2013/387023. Epub 2013 Mar 4. Clin Dev Immunol. 2013. PMID: 23533454 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

