Overcoming antigen masking of anti-amyloidbeta antibodies reveals breaking of B cell tolerance by virus-like particles in amyloidbeta immunized amyloid precursor protein transgenic mice
- PMID: 15186505
- PMCID: PMC442125
- DOI: 10.1186/1471-2202-5-21
Overcoming antigen masking of anti-amyloidbeta antibodies reveals breaking of B cell tolerance by virus-like particles in amyloidbeta immunized amyloid precursor protein transgenic mice
Abstract
Background: In prior work we detected reduced anti-Abeta antibody titers in Abeta-vaccinated transgenic mice expressing the human amyloid precursor protein (APP) compared to nontransgenic littermates. We investigated this observation further by vaccinating APP and nontransgenic mice with either the wild-type human Abeta peptide, an Abeta peptide containing the "Dutch Mutation", E22Q, or a wild-type Abeta peptide conjugated to papillomavirus virus-like particles (VLPs).
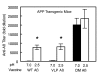
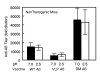
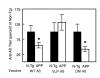
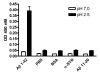
Results: Anti-Abeta antibody titers were lower in vaccinated APP than nontransgenic mice even when vaccinated with the highly immunogenic Abeta E22Q. One concern was that human Abeta derived from the APP transgene might mask anti-Abeta antibodies in APP mice. To test this possibility, we dissociated antigen-antibody complexes by incubation at low pH. The low pH incubation increased the anti-Abeta antibody titers 20-40 fold in APP mice but had no effect in sera from nontransgenic mice. However, even after dissociation, the anti-Abeta titers were still lower in transgenic mice vaccinated with wild-type Abeta or E22Q Abeta relative to non-transgenic mice. Importantly, the dissociated anti-Abeta titers were equivalent in nontransgenic and APP mice after VLP-based vaccination. Control experiments demonstrated that after acid-dissociation, the increased antibody titer did not cross react with bovine serum albumin nor alpha-synuclein, and addition of Abeta back to the dissociated serum blocked the increase in antibody titers.
Conclusions: Circulating human Abeta can interfere with ELISA assay measurements of anti-Abeta titers. The E22Q Abeta peptide vaccine is more immunogenic than the wild-type peptide. Unlike peptide vaccines, VLP-based vaccines against Abeta abrogate the effects of Abeta self-tolerance.
Figures
References
-
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. - DOI - PubMed
-
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. - DOI - PubMed
-
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. - DOI - PubMed
-
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

