Proteomics of protein secretion by Bacillus subtilis: separating the "secrets" of the secretome
- PMID: 15187182
- PMCID: PMC419921
- DOI: 10.1128/MMBR.68.2.207-233.2004
Proteomics of protein secretion by Bacillus subtilis: separating the "secrets" of the secretome
Abstract
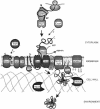
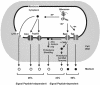
Secretory proteins perform a variety of important "remote-control" functions for bacterial survival in the environment. The availability of complete genome sequences has allowed us to make predictions about the composition of bacterial machinery for protein secretion as well as the extracellular complement of bacterial proteomes. Recently, the power of proteomics was successfully employed to evaluate genome-based models of these so-called secretomes. Progress in this field is well illustrated by the proteomic analysis of protein secretion by the gram-positive bacterium Bacillus subtilis, for which approximately 90 extracellular proteins were identified. Analysis of these proteins disclosed various "secrets of the secretome," such as the residence of cytoplasmic and predicted cell envelope proteins in the extracellular proteome. This showed that genome-based predictions reflect only approximately 50% of the actual composition of the extracellular proteome of B. subtilis. Importantly, proteomics allowed the first verification of the impact of individual secretion machinery components on the total flow of proteins from the cytoplasm to the extracellular environment. In conclusion, proteomics has yielded a variety of novel leads for the analysis of protein traffic in B. subtilis and other gram-positive bacteria. Ultimately, such leads will serve to increase our understanding of virulence factor biogenesis in gram-positive pathogens, which is likely to be of high medical relevance.
Figures



References
-
- Akita, M., S. Sasaki, S. Matsuyama, and S. Mizushima. 1990. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J. Biol. Chem. 265:8162-8169. - PubMed
-
- Alami, M., I. Lüke, S. Deitermann, J. Brunner, and M. Müller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12:937-946. - PubMed
-
- Antelmann, H., E. Darmon, D. Noone, J. W. Veening, H. Westers, S. Bron, O. P. Kuipers, K. M. Devine, M. Hecker, and J. M. van Dijl. 2003. The extracellular proteome of Bacillus subtilis under secretion stress conditions. Mol. Microbiol. 49:143-156. - PubMed
-
- Antelmann, H., H. Tjalsma, B. Voigt, S. Ohlmeier, S. Bron, J. M. van Dijl, and M. Hecker. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 11:14984-14502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

