Diversity in chemotaxis mechanisms among the bacteria and archaea
- PMID: 15187186
- PMCID: PMC419924
- DOI: 10.1128/MMBR.68.2.301-319.2004
Diversity in chemotaxis mechanisms among the bacteria and archaea
Abstract
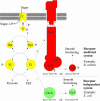
The study of chemotaxis describes the cellular processes that control the movement of organisms toward favorable environments. In bacteria and archaea, motility is controlled by a two-component system involving a histidine kinase that senses the environment and a response regulator, a very common type of signal transduction in prokaryotes. Most insights into the processes involved have come from studies of Escherichia coli over the last three decades. However, in the last 10 years, with the sequencing of many prokaryotic genomes, it has become clear that E. coli represents a streamlined example of bacterial chemotaxis. While general features of excitation remain conserved among bacteria and archaea, specific features, such as adaptational processes and hydrolysis of the intracellular signal CheY-P, are quite diverse. The Bacillus subtilis chemotaxis system is considerably more complex and appears to be similar to the one that existed when the bacteria and archaea separated during evolution, so that understanding this mechanism should provide insight into the variety of mechanisms used today by the broad sweep of chemotactic bacteria and archaea. However, processes even beyond those used in E. coli and B. subtilis have been discovered in other organisms. This review emphasizes those used by B. subtilis and these other organisms but also gives an account of the mechanism in E. coli.
Figures







References
-
- Aizawa, S. I., Zhulin, I. B., L. Marquez-Magana, and G. W. Ordal. 2002. Chemotaxis and motility, p. 437-452. In A. L. Sonenshein, R. Losick, and J. A. Hoch (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
-
- Aksamit, R., and D. E. Koshland, Jr. 1972. A ribose binding protein of Salmonella typhimurium. Biochem. Biophys. Res. Commun. 48:1348-1353. - PubMed
-
- Aksamit, R. R., and D. E. Koshland, Jr. 1974. Identification of the ribose binding protein as the receptor for ribose chemotaxis in Salmonella typhimurium. Biochemistry 13:4473-4478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

