Ionic permeation free energy in gramicidin: a semimicroscopic perspective
- PMID: 15189852
- PMCID: PMC1304257
- DOI: 10.1529/biophysj.103.039214
Ionic permeation free energy in gramicidin: a semimicroscopic perspective
Abstract
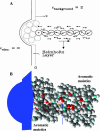
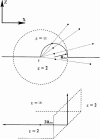
Ion permeation through the gramicidin channel is studied using a model that circumvents two major difficulties inherent to standard simulational methods. It exploits the timescale separation between electronic and structural contributions to dielectric stabilization, accounting for the influence of electronic polarization by embedding the channel in a dielectric milieu that describes this polarization in a mean sense. The explicit mobile moieties are the ion, multipolar waters, and the carbonyls and amides of the peptide backbone. The model treats the influence of aromatic residues and the membrane dipole potential. A new electrical geometry is introduced that treats long-range electrostatics exactly and avoids problems related to periodic boundary conditions. It permits the translocating ion to make a seamless transition from nearby electrolyte to the channel interior. Other degrees of freedom (more distant bulk electrolyte and nonpolar lipid) are treated as dielectric continua. Reasonable permeation free energy profiles are obtained for potassium, rubidium, and cesium; binding wells are shallow and the central barrier is small. Estimated cationic single-channel conductances are smaller than experiment, but only by factors between 2 (rubidium) and 50 (potassium). When applied to chloride the internal barrier is large, with a corresponding miniscule single-channel conductance. The estimated relative single-channel conductances of gramicidin A, B, and C agree well with experiment.
Figures






Similar articles
-
A semi-microscopic Monte Carlo study of permeation energetics in a gramicidin-like channel: the origin of cation selectivity.Biophys J. 1996 Jan;70(1):121-34. doi: 10.1016/S0006-3495(96)79554-1. Biophys J. 1996. PMID: 8770192 Free PMC article.
-
The role of the dielectric barrier in narrow biological channels: a novel composite approach to modeling single-channel currents.Biophys J. 2003 Jun;84(6):3646-61. doi: 10.1016/S0006-3495(03)75095-4. Biophys J. 2003. PMID: 12770873 Free PMC article.
-
Noncontact dipole effects on channel permeation. VI. 5F- and 6F-Trp gramicidin channel currents.Biophys J. 2002 Oct;83(4):1974-86. doi: 10.1016/S0006-3495(02)73959-3. Biophys J. 2002. PMID: 12324416 Free PMC article.
-
Theoretical and computational models of biological ion channels.Q Rev Biophys. 2004 Feb;37(1):15-103. doi: 10.1017/s0033583504003968. Q Rev Biophys. 2004. PMID: 17390604 Review.
-
Permeation in ion channels: the interplay of structure and theory.Trends Neurosci. 2004 Jun;27(6):308-14. doi: 10.1016/j.tins.2004.03.013. Trends Neurosci. 2004. PMID: 15165734 Review.
Cited by
-
Water and ion permeation in bAQP1 and GlpF channels: a kinetic Monte Carlo study.Biophys J. 2004 Dec;87(6):3690-702. doi: 10.1529/biophysj.104.043315. Epub 2004 Sep 17. Biophys J. 2004. PMID: 15377535 Free PMC article.
-
Protein Motifs for Proton Transfers That Build the Transmembrane Proton Gradient.Front Chem. 2021 Jun 15;9:660954. doi: 10.3389/fchem.2021.660954. eCollection 2021. Front Chem. 2021. PMID: 34211960 Free PMC article. Review.
-
Structure based computational assessment of channel properties of assembled ORF-8a from SARS-CoV.Proteins. 2015 Feb;83(2):300-8. doi: 10.1002/prot.24721. Epub 2014 Dec 19. Proteins. 2015. PMID: 25394339 Free PMC article.
-
Energetics of ion permeation, rejection, binding, and block in gramicidin A from free energy simulations.Biophys J. 2006 Jun 1;90(11):3941-50. doi: 10.1529/biophysj.105.074633. Epub 2006 Mar 13. Biophys J. 2006. PMID: 16533834 Free PMC article.
-
On the importance of atomic fluctuations, protein flexibility, and solvent in ion permeation.J Gen Physiol. 2004 Dec;124(6):679-90. doi: 10.1085/jgp.200409111. J Gen Physiol. 2004. PMID: 15572347 Free PMC article.
References
-
- Accardi, A., and C. Miller. 2004. Secondary active transport mediated by a prokaryotic homologue of CIC CI− channels. Nature. 427:803–807. - PubMed
-
- Allen, T. W., O. S. Andersen, and B. Roux. 2003a. Structure of gramicidin A in a lipid bilayer environment determined using molecular dynamics simulations and solid-state NMR. J. Am. Chem. Soc. 125:9868–9877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

