Cholera toxin assault on lipid monolayers containing ganglioside GM1
- PMID: 15189866
- PMCID: PMC1304271
- DOI: 10.1529/biophysj.103.032508
Cholera toxin assault on lipid monolayers containing ganglioside GM1
Abstract
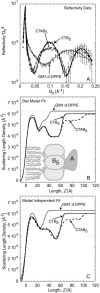
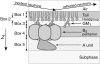
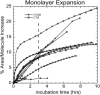
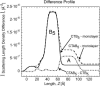
Many bacterial toxins bind to and gain entrance to target cells through specific interactions with membrane components. Using neutron reflectivity, we have characterized the structure of mixed DPPE:GM(1) lipid monolayers before and during the binding of cholera toxin (CTAB(5)) or its B-subunit (CTB(5)). Structural parameters such as the density and thickness of the lipid layer, extension of the GM(1) oligosaccharide headgroup, and orientation and position of the protein upon binding are reported. The density of the lipid layer was found to decrease slightly upon protein binding. However, the A-subunit of the whole toxin is clearly located below the B-pentameric ring, away from the monolayer, and does not penetrate into the lipid layer before enzymatic cleavage. Using Monte Carlo simulations, the observed monolayer expansion was found to be consistent with geometrical constraints imposed on DPPE by multivalent binding of GM(1) by the toxin. Our findings suggest that the mechanism of membrane translocation by the protein may be aided by alterations in lipid packing.
Figures



References
-
- Braslau, A., P. S. Pershan, G. Swislow, B. M. Ocko, and J. Als-Nielsen. 1988. Capillary waves on the surface of simple liquids measured by x-ray reflectivity. Phys. Rev. A. 38:2457–2470. - PubMed
-
- Faller, R., and J. J. de Pablo. 2002. Constant pressure hybrid molecular dynamics-Monte Carlo simulations. J. Chem. Phys. 116:55–59.
-
- Faller, R., and J. J. de Pablo. 2003. Density of states of a binary Lennard-Jones glass. J. Chem. Phys. 119:4405–4408.
-
- Faller, R., and T. L. Kuhl. 2003. Modeling the binding of cholera-toxin to a lipid membrane by a nonadditive two-dimensional hard disk model. Soft Mat. 1:343–352.
-
- Grigera, T. S., and G. Parisi. 2001. Fast Monte Carlo algorithm for supercooled soft spheres. Art. no. 045102. Phys. Rev. E. 6304:5102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

