Interaction of horse heart and thermus thermophilus type c cytochromes with phospholipid vesicles and hydrophobic surfaces
- PMID: 15189883
- PMCID: PMC1304288
- DOI: 10.1529/biophysj.103.025114
Interaction of horse heart and thermus thermophilus type c cytochromes with phospholipid vesicles and hydrophobic surfaces
Abstract
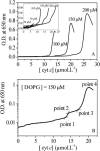
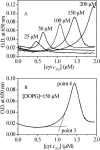
The binding of horse heart cytochrome c (cyt-c) and Thermus thermophilus cytochrome c(552) (cyt-c(552)) to dioleoyl phosphatidylglycerol (DOPG) vesicles was investigated using Fourier transform infrared (FTIR) spectroscopy and turbidity measurements. FTIR spectra revealed that the tertiary structures of both cytochromes became more open when bound to DOPG vesicles, but this was more pronounced for cyt-c. Their secondary structures were unchanged. Turbidity measurements showed important differences in their behavior bound to the negatively charged DOPG vesicles. Both cytochromes caused the liposomes to aggregate and flocculate, but the ways they did so differed. For cyt-c, more than a monolayer was adsorbed onto the liposome surface prior to aggregation due to charge neutralization, whereas cyt c(552) caused aggregation at a protein/lipid ratio well below that required for charge neutralization. Therefore, although cyt-c may cause liposomes to aggregate by electrostatic interaction, cyt-c(552) does not act in this way. FTIR-attenuated total reflection spectroscopy (FTIR-ATR) revealed that cyt-c lost much of its secondary structure when bound to the hydrophobic surface of octadecyltrichlorosilane, whereas cyt-c(552) folds its domains into a beta-structure. This hydrophobic effect may be the key to the difference between the behaviors of the two cytochromes when bound to DOPG vesicles.
Figures




Similar articles
-
FTIR study of the thermal denaturation of alpha-actinin in its lipid-free and dioleoylphosphatidylglycerol-bound states and the central and N-terminal domains of alpha-actinin in D2O.Biochemistry. 1998 Jul 28;37(30):10730-7. doi: 10.1021/bi9800451. Biochemistry. 1998. PMID: 9692963
-
Investigation of secondary and tertiary structural changes of cytochrome c in complexes with anionic lipids using amide hydrogen exchange measurements: an FTIR study.Biophys J. 1993 Dec;65(6):2408-17. doi: 10.1016/S0006-3495(93)81299-2. Biophys J. 1993. PMID: 8312479 Free PMC article.
-
Interaction of cytochrome c with cardiolipin: an infrared spectroscopic study.Biophys Chem. 1995 May;54(3):271-8. doi: 10.1016/0301-4622(94)00151-9. Biophys Chem. 1995. PMID: 7749061
-
Characterization and redox properties of cytochrome c552 from Thermus thermophilus adsorbed on different self-assembled thiol monolayers, used to model the chemical environment of the redox partner.Biopolymers. 2006 Apr 5;81(5):407-18. doi: 10.1002/bip.20432. Biopolymers. 2006. PMID: 16365847
-
Electrostatic and hydrophobic contributions to the folding mechanism of apocytochrome c driven by the interaction with lipid.Biochemistry. 1998 Sep 8;37(36):12588-95. doi: 10.1021/bi980408x. Biochemistry. 1998. PMID: 9730831
Cited by
-
Interactions of apomyoglobin with membranes: mechanisms and effects on heme uptake.Protein Sci. 2007 Mar;16(3):391-400. doi: 10.1110/ps.062531207. Epub 2007 Jan 22. Protein Sci. 2007. PMID: 17242377 Free PMC article.
-
Origin of the conformational heterogeneity of cardiolipin-bound cytochrome C.J Am Chem Soc. 2012 Nov 14;134(45):18713-23. doi: 10.1021/ja307426k. Epub 2012 Nov 2. J Am Chem Soc. 2012. PMID: 23066867 Free PMC article.
-
Versatility of non-native forms of human cytochrome c: pH and micellar concentration dependence.J Biol Inorg Chem. 2013 Jan;18(1):27-38. doi: 10.1007/s00775-012-0946-4. Epub 2012 Oct 16. J Biol Inorg Chem. 2013. PMID: 23070294
-
Cytochrome c/cardiolipin relations in mitochondria: a kiss of death.Free Radic Biol Med. 2009 Jun 1;46(11):1439-53. doi: 10.1016/j.freeradbiomed.2009.03.004. Epub 2009 Mar 12. Free Radic Biol Med. 2009. PMID: 19285551 Free PMC article. Review.
-
Cytochrome c-lipid interactions: new insights from resonance energy transfer.Biophys J. 2010 Sep 22;99(6):1754-63. doi: 10.1016/j.bpj.2010.06.017. Biophys J. 2010. PMID: 20858419 Free PMC article.
References
-
- Baron, M. H., C. De Loze, C. Toniolo, and C. D. Fasman. 1978. Structure in solution of protected homo-oligopeptides of L-valine, L-isoleucine, and L-phenylalanine: an infrared adsorption study. Biopolymers. 17:2225–2239.
-
- Bernad, S., T. Soulimane, and S. Lecomte. 2004. Redox and conformational equilibria of cytochrome c552 from Thermus thermophilus adsorbed on chemically modified silver electrode probed by surface enhanced resonance Raman spectroscopy. J. Raman Spectr. 35:47–54.
-
- Boulkanz, L., N. Balcar, and M. H. Baron. 1995. FT-IR analysis for structural characterization of albumin adsorbed on the reversed-phase support RP-C6. Appl. Spectr. 49:1737–1746.
-
- Boulkanz, L., C. Vidal-Madjar, N. Balcar, and M. H. Baron. 1997. Adsorption mechanism of human serum albumin on a reversed-phase support by kinetic, chromatographic, and FTIR method. J. Coll. Interf. Sci. 188:58–67.
-
- Buschnell, G. W., G. V. Louie, and G. D. Brayer. 1990. High-resolution three-dimensional structure of horse heart cytochrome c. J. Mol. Biol. 214:585–595. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

