Competing hydrophobic and screened-coulomb interactions in hepatitis B virus capsid assembly
- PMID: 15189887
- PMCID: PMC1304292
- DOI: 10.1529/biophysj.104.040055
Competing hydrophobic and screened-coulomb interactions in hepatitis B virus capsid assembly
Abstract
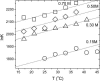
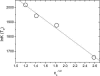
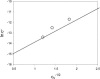
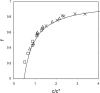
Recent experiments show that, in the range from approximately 15 to 45 degrees C, an increase in the temperature promotes the spontaneous assembly into capsids of the Escherichia coli-expressed coat proteins of hepatitis B virus. Within that temperature interval, an increase in ionic strength up to five times that of standard physiological conditions also acts to promote capsid assembly. To explain both observations we propose an interaction of mean force between the protein subunits that is the sum of an attractive hydrophobic interaction, driving the self-assembly, and a repulsive electrostatic interaction, opposing the self-assembly. We find that the binding strength of the capsid subunits increases with temperature virtually independently of the ionic strength, and that, at fixed temperature, the binding strength increases with the square root of ionic strength. Both predictions are in quantitative agreement with experiment. We point out the similarities of capsid assembly in general and the micellization of surfactants. Finally we make plausible that electrostatic repulsion between the native core subunits of a large class of virus suppresses the formation in vivo of empty virus capsids, that is, without the presence of the charge-neutralizing nucleic acid.
Figures
Similar articles
-
Weak protein-protein interactions are sufficient to drive assembly of hepatitis B virus capsids.Biochemistry. 2002 Oct 1;41(39):11525-31. doi: 10.1021/bi0261645. Biochemistry. 2002. PMID: 12269796
-
A molecular thermodynamic model for the stability of hepatitis B capsids.J Chem Phys. 2014 Jun 21;140(23):235101. doi: 10.1063/1.4882068. J Chem Phys. 2014. PMID: 24952568
-
Hepatitis B Virus Capsids Have Diverse Structural Responses to Small-Molecule Ligands Bound to the Heteroaryldihydropyrimidine Pocket.J Virol. 2016 Mar 28;90(8):3994-4004. doi: 10.1128/JVI.03058-15. Print 2016 Apr. J Virol. 2016. PMID: 26842475 Free PMC article.
-
Role of electrostatic interactions in the assembly of empty spherical viral capsids.Phys Rev E Stat Nonlin Soft Matter Phys. 2007 Dec;76(6 Pt 1):061906. doi: 10.1103/PhysRevE.76.061906. Epub 2007 Dec 12. Phys Rev E Stat Nonlin Soft Matter Phys. 2007. PMID: 18233868
-
Hepatitis B Core Protein Capsids.Subcell Biochem. 2021;96:451-470. doi: 10.1007/978-3-030-58971-4_14. Subcell Biochem. 2021. PMID: 33252740 Review.
Cited by
-
The Role of Packaging Sites in Efficient and Specific Virus Assembly.J Mol Biol. 2015 Jul 31;427(15):2451-2467. doi: 10.1016/j.jmb.2015.05.008. Epub 2015 May 16. J Mol Biol. 2015. PMID: 25986309 Free PMC article.
-
Electrostatic Theory of the Acidity of the Solution in the Lumina of Viruses and Virus-Like Particles.J Phys Chem B. 2023 Mar 16;127(10):2160-2168. doi: 10.1021/acs.jpcb.2c08604. Epub 2023 Mar 7. J Phys Chem B. 2023. PMID: 36881522 Free PMC article.
-
Mechanical deformation of spherical viruses with icosahedral symmetry.Biophys J. 2006 Aug 1;91(3):834-41. doi: 10.1529/biophysj.106.081422. Epub 2006 May 5. Biophys J. 2006. PMID: 16679375 Free PMC article.
-
Role of Protein Charge Density on Hepatitis B Virus Capsid Formation.ACS Omega. 2018 Apr 20;3(4):4384-4391. doi: 10.1021/acsomega.8b00021. eCollection 2018 Apr 30. ACS Omega. 2018. PMID: 31458664 Free PMC article.
-
Size regulation of ss-RNA viruses.Biophys J. 2009 Jan;96(1):9-20. doi: 10.1529/biophysj.108.137489. Biophys J. 2009. PMID: 18931258 Free PMC article.
References
-
- Boström, M., D. R. M. Williams, and B. W. Ninham. 2003. Specific ion effects: the role of co-ions in biology. Europhys. Lett. 63:610–615.
-
- Bringas, R. 1997. Folding and assembly of hepatitis B virus core protein: a new model proposal. J. Struct. Biol. 118:189–196. - PubMed
-
- Broide, M. L., T. M. Tominc, and M. D. Saxowsky. 1996. Using phase transitions to investigate the effect of salts on protein interactions. Phys. Rev. E. 53:6325–6335. - PubMed
-
- Bruinsma, R. F., W. M. Gelbart, D. Reguera, J. Rudnick, and R. Zandi. 2003. Viral self-assembly as a thermodynamic process. Phys. Rev. Lett. 90:248101. - PubMed
-
- Caspar, D. L. D. 1963. Assembly and stability of the Tobacco Mosaic virus particle. Adv. Protein Chem. 18:37–121. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

