Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques
- PMID: 15189902
- PMCID: PMC1304307
- DOI: 10.1529/biophysj.103.035717
Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques
Abstract
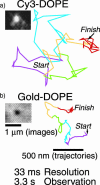
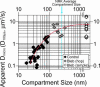
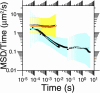
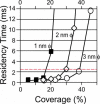
Plasma membrane compartments, delimited by transmembrane proteins anchored to the membrane skeleton (anchored-protein picket model), would provide the membrane with fundamental mosaicism because they would affect the movement of practically all molecules incorporated in the cell membrane. Understanding such basic compartmentalized structures of the cell membrane is critical for further studies of a variety of membrane functions. Here, using both high temporal-resolution single particle tracking and single fluorescent molecule video imaging of an unsaturated phospholipid, DOPE, we found that plasma membrane compartments generally exist in various cell types, including CHO, HEPA-OVA, PtK2, FRSK, HEK293, HeLa, T24 (ECV304), and NRK cells. The compartment size varies from 30 to 230 nm, whereas the average hop rate of DOPE crossing the boundaries between two adjacent compartments ranges between 1 and 17 ms. The probability of passing a compartment barrier when DOPE is already at the boundary is also cell-type dependent, with an overall variation by a factor of approximately 7. These results strongly indicate the necessity for the paradigm shift of the concept on the plasma membrane: from the two-dimensional fluid continuum model to the compartmentalized membrane model in which its constituent molecules undergo hop diffusion over the compartments.
Figures


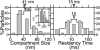


References
-
- Almeida, P. F., W. L. Vaz, and T. E. Thompson. 1992. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 31:7198–7210. - PubMed
-
- Bubb, M. R., I. Spector, B. B. Beyer, and K. M. Fosen. 2000. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J. Biol. Chem. 275:5163–5170. - PubMed
-
- Chang, C.-H., H. Takeuchi, T. Ito, K. Machida, and S. Ohnishi. 1981. Lateral mobility of erythrocyte membrane proteins studied by the fluorescence photobleaching recovery technique. J. Biochem. 90:997–1004. - PubMed
-
- De Mey, J. 1983. Colloidal-gold probes in immunocytochemistry. In Immunocytochemistry (Practical Applications in Pathology and Biology). J. M. Polak and S. van Noorden, editors. WRIGHT PSG, Bristol, UK. 83–112.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

