The proton-driven rotor of ATP synthase: ohmic conductance (10 fS), and absence of voltage gating
- PMID: 15189903
- PMCID: PMC1304308
- DOI: 10.1529/biophysj.103.036962
The proton-driven rotor of ATP synthase: ohmic conductance (10 fS), and absence of voltage gating
Abstract
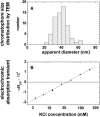
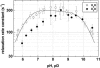
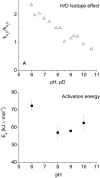
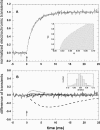
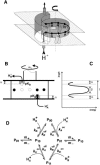
The membrane portion of F(0)F(1)-ATP synthase, F(0), translocates protons by a rotary mechanism. Proton conduction by F(0) was studied in chromatophores of the photosynthetic bacterium Rhodobacter capsulatus. The discharge of a light-induced voltage jump was monitored by electrochromic absorption transients to yield the unitary conductance of F(0). The current-voltage relationship of F(0) was linear from 7 to 70 mV. The current was extremely proton-specific (>10(7)) and varied only slightly ( approximately threefold) from pH 6 to 10. The maximum conductance was approximately 10 fS at pH 8, equivalent to 6240 H(+) s(-1) at 100-mV driving force, which is an order-of-magnitude greater than of coupled F(0)F(1). There was no voltage-gating of F(0) even at low voltage, and proton translocation could be driven by deltapH alone, without voltage. The reported voltage gating in F(0)F(1) is thus attributable to the interaction of F(0) with F(1) but not to F(0) proper. We simulated proton conduction by a minimal rotary model including the rotating c-ring and two relay groups mediating proton exchange between the ring and the respective membrane surface. The data fit attributed pK values of approximately 6 and approximately 10 to these relays, and placed them close to the membrane/electrolyte interface.
Figures


References
-
- Altendorf, K., W. Stalz, J. Greie, and G. Deckers-Hebestreit. 2000. Structure and function of the F0 complex of the ATP synthase from Escherichia coli. J. Exp. Biol. 203:19–28. - PubMed
-
- Althoff, G., H. Lill, and W. Junge. 1989. Proton channel of the chloroplast ATP synthase, CF0: its time- averaged single-channel conductance as function of pH, temperature, isotopic and ionic medium composition. J. Membr. Biol. 108:263–271.
-
- Angevine, C. M., and R. H. Fillingame. 2003. Aqueous access channels in subunit a of rotary ATP synthase. J. Biol. Chem. 278:6066–6074. - PubMed
-
- Ausländer, W., and W. Junge. 1975. Neutral red, a rapid indicator for pH changes in the inner phase of thylakoids. FEBS Lett. 59:310–315.
-
- Baccarini-Melandri, A., H. Gest, and A. S. Pietro. 1970. A coupling factor in bacterial photophosphorylation. J. Biol. Chem. 245:1224–1226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

