Suppressors of an adenylate cyclase deletion in the fission yeast Schizosaccharomyces pombe
- PMID: 15189983
- PMCID: PMC420129
- DOI: 10.1128/EC.3.3.610-619.2004
Suppressors of an adenylate cyclase deletion in the fission yeast Schizosaccharomyces pombe
Abstract
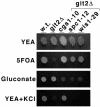
Schizosaccharomyces pombe utilizes two opposing signaling pathways to sense and respond to its nutritional environment. Glucose detection triggers a cyclic AMP signal to activate protein kinase A (PKA), while glucose or nitrogen starvation activates the Spc1/Sty1 stress-activated protein kinase (SAPK). One process controlled by these pathways is fbp1+ transcription, which is glucose repressed. In this study, we isolated strains carrying mutations that reduce high-level fbp1+ transcription conferred by the loss of adenylate cyclase (git2delta), including both wis1- (SAPK kinase) and spc1- (SAPK) mutants. While characterizing the git2delta suppressor strains, we found that the git2delta parental strains are KCl sensitive, though not osmotically sensitive. Of 102 git2delta suppressor strains, 17 strains display KCl-resistant growth and comprise a single linkage group, carrying mutations in the cgs1+ PKA regulatory subunit gene. Surprisingly, some of these mutants are mostly wild type for mating and stationary-phase viability, unlike the previously characterized cgs1-1 mutant, while showing a significant defect in fbp1-lacZ expression. Thus, certain cgs1- mutant alleles dramatically affect some PKA-regulated processes while having little effect on others. We demonstrate that the PKA and SAPK pathways regulate both cgs1+ and pka1+ transcription, providing a mechanism for cross talk between these two antagonistically acting pathways and feedback regulation of the PKA pathway. Finally, strains defective in both the PKA and SAPK pathways display transcriptional regulation of cgs1+ and pka1+, suggesting the presence of a third glucose-responsive signaling pathway.
Copyright 2004 American Society for Microbiology
Figures





References
-
- Caspari, T. 1997. Onset of gluconate-H+ symport in Schizosaccharomyces pombe is regulated by the kinases Wis1 and Pka1, and requires the gti1+ gene product. J. Cell Sci. 110:2599-2608. - PubMed
-
- Caspari, T., and S. Urlinger. 1996. The activity of the gluconate-H+ symporter of Schizosaccharomyces pombe cells is down-regulated by D-glucose and exogenous cAMP. FEBS Lett. 395:272-276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

