Multiple functionally redundant signals mediate targeting to the apicoplast in the apicomplexan parasite Toxoplasma gondii
- PMID: 15189987
- PMCID: PMC420125
- DOI: 10.1128/EC.3.3.663-674.2004
Multiple functionally redundant signals mediate targeting to the apicoplast in the apicomplexan parasite Toxoplasma gondii
Abstract
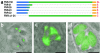
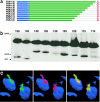
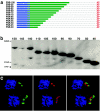
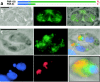
Most species of the protozoan phylum Apicomplexa harbor an endosymbiotic organelle--the apicoplast--acquired when an ancestral parasite engulfed a eukaryotic plastid-containing alga. Several hundred proteins are encoded in the parasite nucleus and are posttranslationally targeted to the apicoplast by a distinctive bipartite signal. The N-terminal 20 to 30 amino acids of nucleus-encoded apicoplast targeted proteins function as a classical signal sequence, mediating entry into the secretory pathway. Cleavage of the signal sequence exposes a transit peptide of variable length (50 to 200 amino acids) that is required for directing proteins to the apicoplast. Although these peptides are enriched in basic amino acids, their structural and functional characteristics are not well understood, which hampers the identification of apicoplast proteins that may constitute novel chemotherapeutic targets. To identify functional domains for a model apicoplast transit peptide, we generated more than 80 deletions and mutations throughout the transit peptide of Toxoplasma gondii ferredoxin NADP+ reductase (TgFNR) and examined the ability of these altered transit peptides to mediate proper targeting and processing of a fluorescent protein reporter. These studies revealed the presence of numerous functional domains. Processing can take place at multiple sites in the protein sequence and may occur outside of the apicoplast lumen. The TgFNR transit peptide contains at least two independent and functionally redundant targeting signals, each of which contains a subdomain that is required for release from or proper sorting within the endoplasmic reticulum. Certain deletion constructs traffic to multiple locations, including the apicoplast periphery, the rhoptries, and the parasitophorous vacuole, suggesting a common thread for targeting to these specialized compartments.
Copyright 2004 American Society for Microbiology
Figures

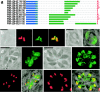
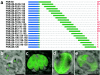

Similar articles
-
N-terminal positively charged amino acids, but not their exact position, are important for apicoplast transit peptide fidelity in Toxoplasma gondii.Mol Biochem Parasitol. 2006 Dec;150(2):192-200. doi: 10.1016/j.molbiopara.2006.08.001. Epub 2006 Aug 28. Mol Biochem Parasitol. 2006. PMID: 16963133
-
Redundant targeting signals of the apicoplast-resident protein TgMnmA in Toxoplasma gondii involve trans-organellar function.Vet Parasitol. 2023 Mar;315:109888. doi: 10.1016/j.vetpar.2023.109888. Epub 2023 Jan 22. Vet Parasitol. 2023. PMID: 36731210
-
Evidence for Golgi-independent transport from the early secretory pathway to the plastid in malaria parasites.Mol Microbiol. 2006 Aug;61(3):614-30. doi: 10.1111/j.1365-2958.2006.05244.x. Epub 2006 Jun 20. Mol Microbiol. 2006. PMID: 16787449
-
Protein trafficking inside Toxoplasma gondii.Traffic. 2008 May;9(5):636-46. doi: 10.1111/j.1600-0854.2008.00713.x. Epub 2008 Mar 4. Traffic. 2008. PMID: 18331382 Review.
-
Protein targeting to the malaria parasite plastid.Traffic. 2008 Feb;9(2):166-75. doi: 10.1111/j.1600-0854.2007.00660.x. Epub 2007 Nov 13. Traffic. 2008. PMID: 17900270 Review.
Cited by
-
Protein Sorting in Plasmodium Falciparum.Life (Basel). 2021 Sep 9;11(9):937. doi: 10.3390/life11090937. Life (Basel). 2021. PMID: 34575086 Free PMC article. Review.
-
A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii.Nat Commun. 2021 Oct 4;12(1):5802. doi: 10.1038/s41467-021-25987-5. Nat Commun. 2021. PMID: 34608145 Free PMC article.
-
Evolving insights into protein trafficking to the multiple compartments of the apicomplexan plastid.J Eukaryot Microbiol. 2009 May-Jun;56(3):214-20. doi: 10.1111/j.1550-7408.2009.00405.x. J Eukaryot Microbiol. 2009. PMID: 19527348 Free PMC article. Review.
-
The apicoplast.Protoplasma. 2011 Oct;248(4):641-50. doi: 10.1007/s00709-010-0250-5. Epub 2010 Dec 17. Protoplasma. 2011. PMID: 21165662 Review.
-
Suggestive evidence for Darwinian Selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii.Eukaryot Cell. 2010 Feb;9(2):228-41. doi: 10.1128/EC.00197-09. Epub 2009 Sep 25. Eukaryot Cell. 2010. PMID: 19783771 Free PMC article.
References
-
- Apt, K. E., L. Zaslavkaia, J. C. Lippmeier, M. Lang, O. Kilian, R. Wetherbee, A. R. Grossman, and P. G. Kroth. 2002. In vivo characterization of diatom multipartite plastid targeting signals. J. Cell Sci. 115:4061-4069. - PubMed
-
- Beckers, C. J., J. F. Dubremetz, O. Mercereau-Puijalon, and K. A. Joiner. 1994. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 127:947-961. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

