Overexpression of sphingosine-1-phosphate lyase or inhibition of sphingosine kinase in Dictyostelium discoideum results in a selective increase in sensitivity to platinum-based chemotherapy drugs
- PMID: 15190000
- PMCID: PMC420124
- DOI: 10.1128/EC.3.3.795-805.2004
Overexpression of sphingosine-1-phosphate lyase or inhibition of sphingosine kinase in Dictyostelium discoideum results in a selective increase in sensitivity to platinum-based chemotherapy drugs
Abstract
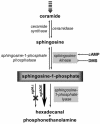
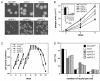
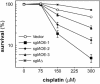
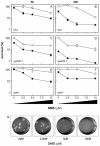
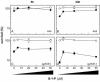
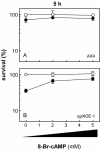
The efficacy of the chemotherapy drug cisplatin is often limited due to resistance of the tumors to the drug, and increasing the potency of cisplatin without increasing its concentration could prove beneficial. A previously characterized Dictyostelium discoideum mutant with increased resistance to cisplatin was defective in the gene encoding sphingosine-1-phosphate (S-1-P) lyase, which catalyzes the breakdown of S-1-P, an important regulatory molecule in cell function and development and in the regulation of cell fate. We hypothesized that the increased resistance to cisplatin was due to an elevation of S-1-P and predicted that lowering levels of S-1-P should increase sensitivity to the drug. We generated three strains that stably overexpress different levels of the S-1-P lyase. The overexpressor strains have reduced growth rate and, confirming the hypothesis, showed an expression-dependent increase in sensitivity to cisplatin. Consistently, treating the cells with D-erythro-N,N,-dimethylsphingosine, a known inhibitor of sphingosine kinase, increased the sensitivity of mutant and parent cells to cisplatin, while addition of exogenous S-1-P or 8-Br-cyclic AMP made the cells more resistant to cisplatin. The increased sensitivity of the overexpressors to cisplatin was also observed with the cisplatin analog carboplatin. In contrast, the response to doxorubicin, 5-flurouracil, or etoposide was unaffected, indicating that the involvement of the sphingolipid metabolic pathway in modulating the response to cisplatin is not part of a global genotoxic stress response. The augmented sensitivity to cisplatin appears to be the result of an intracellular signaling function of S-1-P, because D. discoideum does not appear to have endothelial differentiation growth (EDG/S1P) receptors. Overall, the results show that modulation of the sphingolipid pathway at multiple points can result in increased sensitivity to cisplatin and has the potential for increasing the clinical usefulness of this important drug.
Copyright 2004 American Society for Microbiology
Figures

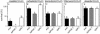
Similar articles
-
Sphingosine kinase regulates the sensitivity of Dictyostelium discoideum cells to the anticancer drug cisplatin.Eukaryot Cell. 2005 Jan;4(1):178-89. doi: 10.1128/EC.4.1.178-189.2005. Eukaryot Cell. 2005. PMID: 15643073 Free PMC article.
-
Sphingosine-1-phosphate lyase regulates sensitivity of human cells to select chemotherapy drugs in a p38-dependent manner.Mol Cancer Res. 2005 May;3(5):287-96. doi: 10.1158/1541-7786.MCR-04-0197. Mol Cancer Res. 2005. PMID: 15886300
-
Lead genetic studies in Dictyostelium discoideum and translational studies in human cells demonstrate that sphingolipids are key regulators of sensitivity to cisplatin and other anticancer drugs.Semin Cell Dev Biol. 2011 Feb;22(1):97-104. doi: 10.1016/j.semcdb.2010.10.005. Epub 2010 Oct 15. Semin Cell Dev Biol. 2011. PMID: 20951822 Review.
-
(Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1.Mol Cancer Res. 2007 Aug;5(8):801-12. doi: 10.1158/1541-7786.MCR-07-0100. Mol Cancer Res. 2007. PMID: 17699106
-
Inhibitors of sphingosine-1-phosphate metabolism (sphingosine kinases and sphingosine-1-phosphate lyase).Chem Phys Lipids. 2016 May;197:69-81. doi: 10.1016/j.chemphyslip.2015.07.007. Epub 2015 Jul 19. Chem Phys Lipids. 2016. PMID: 26200919 Review.
Cited by
-
S1P Lyase Regulation of Thymic Egress and Oncogenic Inflammatory Signaling.Mediators Inflamm. 2017;2017:7685142. doi: 10.1155/2017/7685142. Epub 2017 Dec 3. Mediators Inflamm. 2017. PMID: 29333002 Free PMC article. Review.
-
Sphingosine kinase regulates the sensitivity of Dictyostelium discoideum cells to the anticancer drug cisplatin.Eukaryot Cell. 2005 Jan;4(1):178-89. doi: 10.1128/EC.4.1.178-189.2005. Eukaryot Cell. 2005. PMID: 15643073 Free PMC article.
-
Sphingosine-1-phosphate lyase in development and disease: sphingolipid metabolism takes flight.Biochim Biophys Acta. 2008 Sep;1781(9):448-58. doi: 10.1016/j.bbalip.2008.05.005. Epub 2008 Jun 17. Biochim Biophys Acta. 2008. PMID: 18558101 Free PMC article. Review.
-
Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function.Adv Enzyme Regul. 2010;50(1):349-62. doi: 10.1016/j.advenzreg.2009.10.024. Epub 2009 Nov 13. Adv Enzyme Regul. 2010. PMID: 19914275 Free PMC article. Review. No abstract available.
-
Hair Cell Loss Induced by Sphingosine and a Sphingosine Kinase Inhibitor in the Rat Cochlea.Neurotox Res. 2016 Jan;29(1):35-46. doi: 10.1007/s12640-015-9563-7. Neurotox Res. 2016. PMID: 26472207
References
-
- Alexander, H., A. N. Vomund, and S. Alexander. 2003. Viability assay for Dictyostelium for use in drug studies. BioTechniques 35:464-470. - PubMed
-
- Arcamone, F. 1981. Doxorubicin anticancer antibiotics, medicinal chemistry, a series of monographs, vol. 17. Academic Press, New York, N.Y.
-
- Burger, H., A. Capello, P. W. Schenk, G. Stoter, J. Brouwer, and K. Nooter. 2000. A genome-wide screening in Saccharomyces cerevisiae for genes that confer resistance to the anticancer agent cisplatin. Biochem. Biophys. Res. Commun. 269:767-774. - PubMed
-
- Cuvillier, O., G. Pirianov, B. Kleuser, P. G. Vanek, O. A. Coso, S. Gutkind, and S. Spiegel. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381:800-803. - PubMed
-
- Doroshow, J. H. 1996. Anthracyclines and anthracenediones, p. 500-537. In B. A. Chabner and D. L. M. Longo (ed.), Cancer chemotherapy and biotherapy: principles and practice, 3rd ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

