Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice
- PMID: 15190010
- PMCID: PMC2431460
- DOI: 10.1093/hmg/ddh174
Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice
Abstract
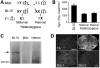
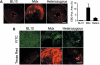
Gene therapy holds great promise for curing Duchenne muscular dystrophy (DMD), the most common fatal inherited childhood muscle disease. Success of DMD gene therapy depends upon functional improvement in both skeletal and cardiac muscle. Numerous gene transfer studies have been performed to correct skeletal muscle pathology, yet little is known about cardiomyopathy gene therapy. Since complete transduction of the entire heart is an impractical goal, it becomes critical to determine the minimal level of correction needed for successful DMD cardiomyopathy gene therapy. To address this question, we generated heterozygous mice that persistently expressed the full-length dystrophin gene in 50% of the cardiomyocytes of mdx mice, a model for DMD. We questioned whether dystrophin expression in half of the heart cells was sufficient to prevent stress-induced cardiomyopathy. Heart function of mdx mouse is normal in the absence of external stress. To determine the therapeutic effect, we challenged 3-month-old mice with beta-isoproterenol. Cardiomyocyte sarcolemma integrity was significantly impaired in mdx but not in heterozygous and C57Bl/10 mice. Importantly, in vivo closed-chest hemodynamic assays revealed normal left ventricular function in beta-isoproterenol-stimulated heterozygous mice. Since the expression profile in the heterozygous mice mimicked viral transduction, we conclude that gene therapy correction in 50% of the heart cells may be sufficient to treat cardiomyopathy in mdx mice. This finding may also apply to the gene therapy of other inherited cardiomyopathies.
Figures


Similar articles
-
Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy.Hum Mol Genet. 2006 Oct 15;15 Spec No 2(SPEC):R253-61. doi: 10.1093/hmg/ddl180. Hum Mol Genet. 2006. PMID: 16987891 Free PMC article. Review.
-
Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart.Circulation. 2003 Sep 30;108(13):1626-32. doi: 10.1161/01.CIR.0000089371.11664.27. Epub 2003 Sep 2. Circulation. 2003. PMID: 12952841 Free PMC article.
-
Acute AT1R blockade prevents isoproterenol-induced injury in mdx hearts.J Mol Cell Cardiol. 2019 Mar;128:51-61. doi: 10.1016/j.yjmcc.2019.01.013. Epub 2019 Jan 19. J Mol Cell Cardiol. 2019. PMID: 30664850 Free PMC article.
-
AAV micro-dystrophin gene therapy alleviates stress-induced cardiac death but not myocardial fibrosis in >21-m-old mdx mice, an end-stage model of Duchenne muscular dystrophy cardiomyopathy.J Mol Cell Cardiol. 2012 Aug;53(2):217-22. doi: 10.1016/j.yjmcc.2012.05.002. Epub 2012 May 12. J Mol Cell Cardiol. 2012. PMID: 22587991 Free PMC article.
-
Modeling Duchenne Muscular Dystrophy Cardiomyopathy with Patients' Induced Pluripotent Stem-Cell-Derived Cardiomyocytes.Int J Mol Sci. 2023 May 12;24(10):8657. doi: 10.3390/ijms24108657. Int J Mol Sci. 2023. PMID: 37240001 Free PMC article. Review.
Cited by
-
Cardiac Involvement in Dystrophin-Deficient Females: Current Understanding and Implications for the Treatment of Dystrophinopathies.Genes (Basel). 2020 Jul 8;11(7):765. doi: 10.3390/genes11070765. Genes (Basel). 2020. PMID: 32650403 Free PMC article. Review.
-
Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy.Hum Mol Genet. 2006 Oct 15;15 Spec No 2(SPEC):R253-61. doi: 10.1093/hmg/ddl180. Hum Mol Genet. 2006. PMID: 16987891 Free PMC article. Review.
-
Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy.Dis Model Mech. 2015 Mar;8(3):195-213. doi: 10.1242/dmm.018424. Dis Model Mech. 2015. PMID: 25740330 Free PMC article. Review.
-
Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice.Hum Gene Ther. 2008 Aug;19(8):851-6. doi: 10.1089/hum.2008.058. Hum Gene Ther. 2008. PMID: 18666839 Free PMC article.
-
Age-matched comparison reveals early electrocardiography and echocardiography changes in dystrophin-deficient dogs.Neuromuscul Disord. 2011 Jul;21(7):453-61. doi: 10.1016/j.nmd.2011.03.010. Epub 2011 May 13. Neuromuscul Disord. 2011. PMID: 21570848 Free PMC article.
References
-
- Emery AE. Some unanswered questions in Duchenne muscular dystrophy. Neuromuscul. Disord. 1994;4:301–303. - PubMed
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Cox GF, Kunkel LM. Dystrophies and heart disease. Curr. Opin. Cardiol. 1997;12:329–343. - PubMed
-
- Melacini P, Fanin M, Danieli GA, Villanova C, Martinello F, Miorin M, Freda MP, Miorelli M, Mostacciuolo ML, Fasoli G, et al. Myocardial involvement is very frequent among patients affected with subclinical Becker's muscular dystrophy. Circulation. 1996;94:3168–3175. - PubMed
-
- Towbin JA, Hejtmancik JF, Brink P, Gelb B, Zhu XM, Chamberlain JS, McCabe ER, Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

