Microdomains for dopamine volume neurotransmission in primate prefrontal cortex
- PMID: 15190100
- PMCID: PMC6729299
- DOI: 10.1523/JNEUROSCI.0195-04.2004
Microdomains for dopamine volume neurotransmission in primate prefrontal cortex
Abstract
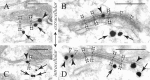
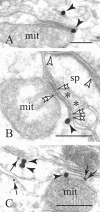
The explicit yet enigmatic involvement of dopamine in cortical physiology is in part volumetric (beyond the synapse), as is apparently the action of neuroleptics targeting dopamine receptors. The notion that nonsynaptic neuronal membranes would translate extracellular dopamine into receptor-specific spatiotemporal downstream signaling, similar to the chemical synapse, is intriguing. Here, we report that dopamine D5 (but not D1 or D2) receptors in the perisomatic plasma membrane of prefrontal cortical neurons form discrete and exclusively extrasynaptic microdomains with inositol 1,4,5-trisphosphate-gated calcium stores of subsurface cisterns and mitochondria. These findings introduce a novel dopaminoceptive substratum in the brain and a unique D5 receptor-specific signaling paradigm.
Figures





Similar articles
-
Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse.J Neurosci. 2005 Feb 2;25(5):1260-7. doi: 10.1523/JNEUROSCI.3436-04.2005. J Neurosci. 2005. PMID: 15689564 Free PMC article.
-
Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: coexpression in dendritic spines and axon terminals.J Comp Neurol. 2008 Jun 20;508(6):893-905. doi: 10.1002/cne.21710. J Comp Neurol. 2008. PMID: 18399540 Free PMC article.
-
Dopamine receptors differentially enhance rule coding in primate prefrontal cortex neurons.Neuron. 2014 Dec 17;84(6):1317-28. doi: 10.1016/j.neuron.2014.11.012. Epub 2014 Dec 4. Neuron. 2014. PMID: 25482027
-
Prefrontal dopamine in associative learning and memory.Neuroscience. 2014 Dec 12;282:217-29. doi: 10.1016/j.neuroscience.2014.09.026. Epub 2014 Sep 18. Neuroscience. 2014. PMID: 25241063 Free PMC article. Review.
-
PET neuroimaging of extrastriatal dopamine receptors and prefrontal cortex functions.J Physiol Paris. 2013 Dec;107(6):503-9. doi: 10.1016/j.jphysparis.2013.07.001. Epub 2013 Jul 12. J Physiol Paris. 2013. PMID: 23851135 Review.
Cited by
-
Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia.Cereb Cortex. 2013 Jul;23(7):1643-54. doi: 10.1093/cercor/bhs152. Epub 2012 Jun 12. Cereb Cortex. 2013. PMID: 22693343 Free PMC article.
-
Major vault protein is expressed along the nucleus-neurite axis and associates with mRNAs in cortical neurons.Cereb Cortex. 2009 Jul;19(7):1666-77. doi: 10.1093/cercor/bhn203. Epub 2008 Nov 21. Cereb Cortex. 2009. PMID: 19029061 Free PMC article.
-
cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):5036-41. doi: 10.1073/pnas.1322360111. Epub 2014 Mar 18. Proc Natl Acad Sci U S A. 2014. PMID: 24707050 Free PMC article.
-
Chronic cocaine disrupts mesocortical learning mechanisms.Brain Res. 2015 Dec 2;1628(Pt A):88-103. doi: 10.1016/j.brainres.2015.02.003. Epub 2015 Feb 20. Brain Res. 2015. PMID: 25704202 Free PMC article. Review.
-
Pharmacology of signaling induced by dopamine D(1)-like receptor activation.Pharmacol Ther. 2010 Oct;128(1):37-60. doi: 10.1016/j.pharmthera.2010.05.003. Epub 2010 Jun 12. Pharmacol Ther. 2010. PMID: 20547182 Free PMC article. Review.
References
-
- Agnati LF, Bjelke B, Fuxe K (1995a) Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology. Med Res Rev 15: 33-45. - PubMed
-
- Agnati LF, Zoli M, Strömberg I, Fuxe K (1995b) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69: 711-726. - PubMed
-
- Agnati LF, Fuxe K, Nicholson C, Syková E (2000) Progress in brain research, Vol 125. Volume transmission revisited. Amsterdam: Elsevier.
-
- Alonso-Nanclares L, Minelli A, Melone M, Edwards RH, DeFelipe J, Conti F (2004) Perisomatic glutamatergic axon terminals: a novel feature of cortical synaptology revealed by vesicular glutamate transporter 1 immunostaining. Neuroscience 123: 547-556. - PubMed
-
- Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR (1997) Cellular distribution of the rat D1B receptor in central nervous system using anti-receptor antisera. Brain Res 746: 141-150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
