Using a hybrid neural system to reveal regulation of neuronal network activity by an intrinsic current
- PMID: 15190116
- PMCID: PMC6729308
- DOI: 10.1523/JNEUROSCI.4449-03.2004
Using a hybrid neural system to reveal regulation of neuronal network activity by an intrinsic current
Abstract
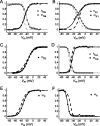
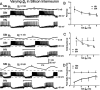
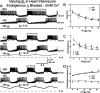
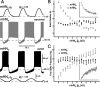
The generation of rhythmic patterns by neuronal networks is a complex phenomenon, relying on the interaction of numerous intrinsic and synaptic currents, as well as modulatory agents. To investigate the functional contribution of an individual ionic current to rhythmic pattern generation in a network, we constructed a hybrid system composed of a silicon model neuron and a heart interneuron from the heartbeat timing network of the medicinal leech. When the model neuron and a heart interneuron are connected by inhibitory synapses, they produce rhythmic activity similar to that observed in the heartbeat network. We focused our studies on investigating the functional role of the hyperpolarization-activated inward current (I(h)) on the rhythmic bursts produced by the network. By introducing changes in both the model and the heart interneuron, we showed that I(h) determines both the period of rhythmic bursts and the balance of activity between the two sides of the network, because the amount and the activation/deactivation time constant of I(h) determines the length of time that a neuron spends in the inhibited phase of its burst cycle. Moreover, we demonstrated that the model neuron is an effective replacement for a heart interneuron and that changes made in the model can accurately mimic similar changes made in the living system. Finally, we used a previously developed mathematical model (Hill et al. 2001) of two mutually inhibitory interneurons to corroborate these findings. Our results demonstrated that this hybrid system technique is advantageous for investigating neuronal properties that are inaccessible with traditional techniques.
Figures







Similar articles
-
Hybrid systems analysis of the control of burst duration by low-voltage-activated calcium current in leech heart interneurons.J Neurophysiol. 2006 Dec;96(6):2857-67. doi: 10.1152/jn.00582.2006. Epub 2006 Aug 30. J Neurophysiol. 2006. PMID: 16943313
-
Modeling the leech heartbeat elemental oscillator. I. Interactions of intrinsic and synaptic currents.J Comput Neurosci. 1995 Sep;2(3):215-35. doi: 10.1007/BF00961435. J Comput Neurosci. 1995. PMID: 8521288
-
Heartbeat control in the medicinal leech: a model system for understanding the origin, coordination, and modulation of rhythmic motor patterns.J Neurobiol. 1995 Jul;27(3):390-402. doi: 10.1002/neu.480270311. J Neurobiol. 1995. PMID: 7673897 Review.
-
Phase relationships between segmentally organized oscillators in the leech heartbeat pattern generating network.J Neurophysiol. 2002 Mar;87(3):1572-85. doi: 10.1152/jn.00336.2001. J Neurophysiol. 2002. PMID: 11877527
-
Neural control of heartbeat in the leech and in some other invertebrates.Physiol Rev. 1979 Jan;59(1):101-36. doi: 10.1152/physrev.1979.59.1.101. Physiol Rev. 1979. PMID: 220645 Review.
Cited by
-
Patterns of presynaptic activity and synaptic strength interact to produce motor output.J Neurosci. 2011 Nov 30;31(48):17555-71. doi: 10.1523/JNEUROSCI.4723-11.2011. J Neurosci. 2011. PMID: 22131417 Free PMC article.
-
Endogenous and half-center bursting in morphologically inspired models of leech heart interneurons.J Neurophysiol. 2006 Oct;96(4):2089-106. doi: 10.1152/jn.00025.2006. Epub 2006 Jun 7. J Neurophysiol. 2006. PMID: 16760353 Free PMC article.
-
Multistability of bursting rhythms in a half-center oscillator and the protective effects of synaptic inhibition.Front Cell Neurosci. 2024 Sep 17;18:1395026. doi: 10.3389/fncel.2024.1395026. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39355175 Free PMC article.
-
Bursting Dynamics Based on the Persistent Na+ and Na+/K+ Pump Currents: A Dynamic Clamp Approach.eNeuro. 2023 Aug 18;10(8):ENEURO.0331-22.2023. doi: 10.1523/ENEURO.0331-22.2023. Print 2023 Aug. eNeuro. 2023. PMID: 37433684 Free PMC article.
-
Comodulation of h- and Na+/K+ Pump Currents Expands the Range of Functional Bursting in a Central Pattern Generator by Navigating between Dysfunctional Regimes.J Neurosci. 2021 Jul 28;41(30):6468-6483. doi: 10.1523/JNEUROSCI.0158-21.2021. Epub 2021 Jun 8. J Neurosci. 2021. PMID: 34103361 Free PMC article.
References
-
- Bal T, McCormick DA (1997) Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J Neurophysiol 77: 3145-3156. - PubMed
-
- Bower JM, Beeman D (1998) The book of GENESIS, Ed 2: exploring realistic neural models with the GEneral NEural SImulation System. New York: Springer-Verlag.
-
- Calabrese RL, Nadim F, Olsen ØH (1995) Heartbeat control in the medicinal leech: a model system for understanding the origin, coordination, and modulation of rhythmic motor patterns. J Neurobiol 27: 390-402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
