Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis
- PMID: 15192152
- PMCID: PMC438990
- DOI: 10.1073/pnas.0403160101
Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis
Abstract
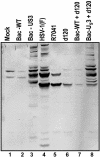
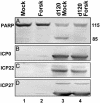
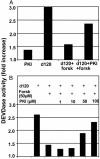
Herpes simplex virus 1 encodes at least four genes whose functions include blocking apoptosis induced by exogenous agents (e.g., sorbitol, Fas ligand, and BAD protein) or replication-incompetent mutants (e.g., the d120 mutant lacking both copies of the alpha 4 gene). U(S)3, one of these four genes, encodes a serine-threonine kinase that has been demonstrated to block apoptosis induced by proapoptotic cellular proteins or by the d120 mutant. The amino acid context of serine-threonine phosphorylated by U(S)3 is similar to that of the cAMP-dependent protein kinase PKA. We report that (i) the pattern of proteins phosphorylated by U(S)3 in transduced cells or in cells infected with WT virus overlaps that of phosphoproteins targeted by PKA, (ii) activation of PKA blocks apoptosis induced by d120 mutant or by BAD protein independently of U(S)3, (iii) U(S)3 protein kinase phosphorylates peptides containing the serine or threonine targeted by PKA including that present in the regulatory type II alpha subunit of PKA, and (iv) in WT virus-infected cells the regulatory type II alpha subunit is phosphorylated in a U(S)3-dependent manner. We conclude that a major determinant of the antiapoptotic activity of the U(S)3 protein kinase is the phosphorylation of PKA substrates by either or both enzymes.
Figures
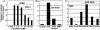


References
-
- Jerome, K. R., Chen, Z., Lang, R., Torres, M. R., Hofmeister, J., Smith, S., Fox, R., Froelich, C. J. & Corey, L. (2001) J. Immunol. 167, 3928-3935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

