DNA damage checkpoint kinase Chk2 triggers replicative senescence
- PMID: 15192702
- PMCID: PMC449769
- DOI: 10.1038/sj.emboj.7600259
DNA damage checkpoint kinase Chk2 triggers replicative senescence
Abstract
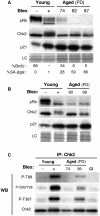
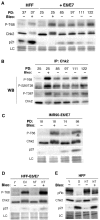
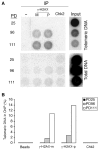
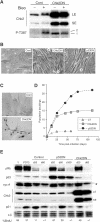
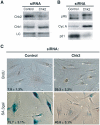
Telomere shortening in normal human cells causes replicative senescence, a p53-dependent growth arrest state, which is thought to represent an innate defence against tumour progression. However, although it has been postulated that critical telomere loss generates a 'DNA damage' signal, the signalling pathway(s) that alerts cells to short dysfunctional telomeres remains only partially defined. We show that senescence in human fibroblasts is associated with focal accumulation of gamma-H2AX and phosphorylation of Chk2, known mediators of the ataxia-telangiectasia mutated regulated signalling pathway activated by DNA double-strand breaks. Both these responses increased in cells grown beyond senescence through inactivation of p53 and pRb, indicating that they are driven by continued cell division and not a consequence of senescence. gamma-H2AX (though not Chk2) was shown to associate directly with telomeric DNA. Furthermore, inactivation of Chk2 in human fibroblasts led to a fall in p21(waf1) expression and an extension of proliferative lifespan, consistent with failure to activate p53. Thus, Chk2 forms an essential component of a common pathway signalling cell cycle arrest in response to both telomere erosion and DNA damage.
Figures


References
-
- Baird DM, Rowson J, Wynford-Thomas D, Kipling D (2003) Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet 33: 203–207 - PubMed
-
- Bartek J, Falck J, Lukas J (2001) CHK2 kinase—a busy messenger. Nat Rev Mol Cell Biol 2: 877–886 - PubMed
-
- Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, Birch JM, Li FP, Garber JE, Haber DA (1999) Heterozygous germ line hCHK2 mutations in Li–Fraumeni syndrome. Science 286: 2528–2531 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

