Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT
- PMID: 15192708
- PMCID: PMC449778
- DOI: 10.1038/sj.emboj.7600268
Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT
Abstract
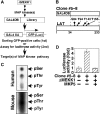
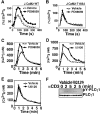
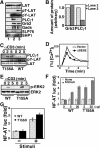
Mitogen-activated protein kinase (MAPK) cascades are involved in a variety of cellular responses including proliferation, differentiation, and apoptosis. We have developed an expression screening method to detect in vivo substrates of MAPKs in mammalian cells, and identified a membrane protein, linker for activation of T cells (LAT), as an MAPK target. LAT, an adapter protein essential for T-cell signaling, is phosphorylated at its Thr 155 by ERK in response to T-cell receptor stimulation. Thr 155 phosphorylation reduces the ability of LAT to recruit PLCgamma1 and SLP76, leading to attenuation of subsequent downstream events such as [Ca2+]i mobilization and activation of the ERK pathway. Our data reveal a new role for MAPKs in a negative feedback loop in T-cell activation via threonine phosphorylation of LAT.
Figures

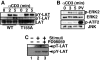

Similar articles
-
Serine residues in the LAT adaptor are essential for TCR-dependent signal transduction.J Leukoc Biol. 2011 Jan;89(1):63-73. doi: 10.1189/jlb.0509342. Epub 2010 Oct 12. J Leukoc Biol. 2011. PMID: 20940326
-
Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes.Mol Cell Biol. 2001 Oct;21(20):6939-50. doi: 10.1128/MCB.21.20.6939-6950.2001. Mol Cell Biol. 2001. PMID: 11564877 Free PMC article.
-
Evidence of LAT as a dual substrate for Lck and Syk in T lymphocytes.Leuk Res. 2007 Apr;31(4):541-5. doi: 10.1016/j.leukres.2006.07.010. Epub 2006 Aug 30. Leuk Res. 2007. PMID: 16938345
-
LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways.Bioessays. 2004 Jan;26(1):61-7. doi: 10.1002/bies.10384. Bioessays. 2004. PMID: 14696041 Review.
-
LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways.Sci STKE. 2000 Dec 19;2000(63):re1. doi: 10.1126/stke.2000.63.re1. Sci STKE. 2000. PMID: 11752630 Review.
Cited by
-
Regulating the discriminatory response to antigen by T-cell receptor.Biosci Rep. 2022 Mar 31;42(3):BSR20212012. doi: 10.1042/BSR20212012. Biosci Rep. 2022. PMID: 35260878 Free PMC article. Review.
-
IL-23 signaling regulation of pro-inflammatory T-cell migration uncovered by phosphoproteomics.PLoS Biol. 2020 Mar 23;18(3):e3000646. doi: 10.1371/journal.pbio.3000646. eCollection 2020 Mar. PLoS Biol. 2020. PMID: 32203518 Free PMC article.
-
T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1549-54. doi: 10.1073/pnas.1017340108. Epub 2011 Jan 4. Proc Natl Acad Sci U S A. 2011. PMID: 21205892 Free PMC article.
-
Regulation of lymphocyte development and activation by the LAT family of adapter proteins.Immunol Rev. 2009 Nov;232(1):72-83. doi: 10.1111/j.1600-065X.2009.00828.x. Immunol Rev. 2009. PMID: 19909357 Free PMC article. Review.
-
Negative feedback regulation of the ERK1/2 MAPK pathway.Cell Mol Life Sci. 2016 Dec;73(23):4397-4413. doi: 10.1007/s00018-016-2297-8. Epub 2016 Jun 24. Cell Mol Life Sci. 2016. PMID: 27342992 Free PMC article. Review.
References
-
- Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M (2002) Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296: 2036–2040 - PubMed
-
- Ahn NG, Seger R, Krebs EG (1992) The mitogen-activated protein kinase activator. Curr Opin Cell Biol 4: 992–999 - PubMed
-
- Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM (1995) Selective requirement for MAP kinase activation in thymocyte differentiation. Nature 373: 620–623 - PubMed
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270: 27489–27494 - PubMed
-
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR (2003) Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5: 647–654 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

