PTEN function: how normal cells control it and tumour cells lose it
- PMID: 15193142
- PMCID: PMC1133909
- DOI: 10.1042/BJ20040825
PTEN function: how normal cells control it and tumour cells lose it
Abstract
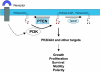
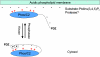
The PTEN (phosphatase and tensin homologue deleted on chromosome 10) tumour suppressor is a PI (phosphoinositide) 3-phosphatase that can inhibit cellular proliferation, survival and growth by inactivating PI 3-kinase-dependent signalling. It also suppresses cellular motility through mechanisms that may be partially independent of phosphatase activity. PTEN is one of the most commonly lost tumour suppressors in human cancer, and its deregulation is also implicated in several other diseases. Here we discuss recent developments in our understanding of how the cellular activity of PTEN is regulated, and the closely related question of how this activity is lost in tumours. Cellular PTEN function appears to be regulated by controlling both the expression of the enzyme and also its activity through mechanisms including oxidation and phosphorylation-based control of non-substrate membrane binding. Therefore mutation of PTEN in tumours disrupts not only the catalytic function of PTEN, but also its regulatory aspects. However, although mutation of PTEN is uncommon in many human tumour types, loss of PTEN expression seems to be more frequent. It is currently unclear how these tumours lose PTEN expression in the absence of mutation, and while some data implicate other potential tumour suppressors and oncogenes in this process, this area seems likely to be a key focus of future research.
Figures

References
-
- Vanhaesebroeck B., Leevers S. J., Ahmadi K., Timms J., Katso R., Driscoll P. C., Woscholski R., Parker P. J., Waterfield M. D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. - PubMed
-
- Foster F. M., Traer C. J., Abraham S. M., Fry M. J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003;116:3037–3040. - PubMed
-
- Katso R., Okkenhaug K., Ahmadi K., White S., Timms J., Waterfield M. D. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001;17:615–675. - PubMed
-
- Cantley L. C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Cantrell D. A. Phosphoinositide 3-kinase signalling pathways. J. Cell Sci. 2001;114:1439–1445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

