Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells
- PMID: 15194426
- PMCID: PMC3843129
- DOI: 10.1016/j.yexcr.2004.02.017
Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells
Abstract
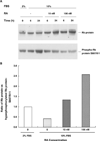
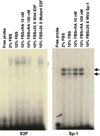
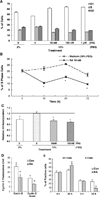
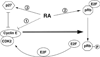
All-trans-retinoic acid (RA), a natural metabolite of retinol, carries out most of the biological activities of vitamin A and is required for normal growth, cell differentiation, and immune functions. In the present studies, THP-1 human monocytes were used to investigate the mechanisms by which RA may regulate progression through the G1/S phase of the cell cycle. Physiological concentrations of all-trans-RA reduced the levels of cyclin E mRNA by 6 h and reduced cyclin E protein in a dose- and time-dependent manner. Similar reductions were observed for the retinoic acid receptor RARalpha and RXRalpha proteins. Concomitantly, RA increased the level of the cyclin-dependent kinase inhibitor p27 (Kip-1). The levels of retinoblastoma mRNA and protein (pRb) were also increased, while the proportion of hyperphosphorylated (phosphoserine 807/811) pRb was markedly reduced. Overall, RA increased the functionality of pRb as an inhibitor of cell cycle progression. Furthermore, RA reduced the binding activity of the transcription factor E2F to its core DNA element. Retinoic acid-induced changes in cell cycle-related proteins occurred in 4-6 h, including reduced cyclin E expression in bromodeoxyuridine (BrdU)-labeled cells, before the onset of cell differentiation as indicated by an increase in the percentage of G1 phase cells and a reduction in S phase cells at 24 h. The expression of CD11b, a cell surface marker of macrophage-like differentiation was increased by RA, as was phagocytic activity. The multiple effects of RA on cell cycle progression may help to explain its well-documented ability to induce the differentiation of THP-1 cells, and thereby to enhance macrophage-like immune functions.
Figures



References
-
- Soderlund MB, Sjoberg A, Svard G, Fex G, Nilsson-Ehle P. Biological variation of retinoids in man. Scand. J. Clin. Lab. Invest. 2002;62:511–519. - PubMed
-
- Altucci L, Gronemeyer H. Nuclear receptors in cell life and death. Trends Endocrinol. Metab. 2001;12:460–468. - PubMed
-
- Wei LN. Retinoid receptors and their coregulators. Annu. Rev. Pharmacol. Toxicol. 2003;43:47–72. - PubMed
-
- Zile MH, Cullum ME, Roltsch IA, DeHoog JV, Welsch CW. Effect of moderate vitamin A supplementation and lack of dietary vitamin A on the development of mammary tumors in female rats treated with low carcinogenic dose levels of 7,12-dimethylbenz(a)anthracene. Cancer Res. 1986;46:3495–3503. - PubMed
-
- Ghosh J, Das S. A study on the effect of vitamin A deficiency and supplementation on tumorigenesis in mice. Neoplasma. 1988;35:41–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

