Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro
- PMID: 15194650
- PMCID: PMC1774091
- DOI: 10.1136/gut.2003.026831
Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro
Abstract
Background: Overexpression of tissue plasminogen activator (t-PA) in pancreatic cancer cells promotes invasion and proliferation in vitro and tumour growth and angiogenesis in vivo.
Aims: To understand the mechanisms by which t-PA favours cancer progression, we analysed the surface membrane proteins responsible for binding specifically t-PA and studied the contribution of this interaction to the t-PA promoted invasion of pancreatic cancer cells.
Methods: The ability of t-PA to activate plasmin and a fluorogenic plasmin substrate was used to analyse the nature of the binding of active t-PA to cell surfaces. Specific binding was determined in two pancreatic cancer cell lines (SK-PC-1 and PANC-1), and complex formation analysed by co-immunoprecipitation experiments and co-immunolocalisation in tumours. The functional role of the interaction was studied in Matrigel invasion assays.
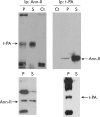
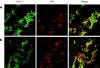
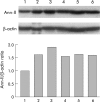
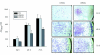
Results: t-PA bound to PANC-1 and SK-PC-1 cells in a specific and saturable manner while maintaining its activity. This binding was competitively inhibited by specific peptides interfering with the interaction of t-PA with annexin II. The t-PA/annexin II interaction on pancreatic cancer cells was also supported by co-immunoprecipitation assays using anti-t-PA antibodies and, reciprocally, with antiannexin II antibodies. In addition, confocal microscopy showed t-PA and annexin II colocalisation in tumour tissues. Finally, disruption of the t-PA/annexin II interaction by a specific hexapeptide significantly decreased the invasive capacity of SK-PC-1 cells in vitro.
Conclusion: t-PA specifically binds to annexin II on the extracellular membrane of pancreatic cancer cells where it activates local plasmin production and tumour cell invasion. These findings may be clinically relevant for future therapeutic strategies based on specific drugs that counteract the activity of t-PA or its receptor annexin II, or their interaction at the surface level.
Figures
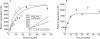
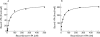
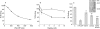
Similar articles
-
The plasminogen activator system in pancreas cancer: role of t-PA in the invasive potential in vitro.Oncogene. 1998 Feb 5;16(5):625-33. doi: 10.1038/sj.onc.1201564. Oncogene. 1998. PMID: 9482108
-
Tissue plasminogen activator is required for the growth, invasion, and angiogenesis of pancreatic tumor cells.Gastroenterology. 2002 Mar;122(3):806-19. doi: 10.1053/gast.2002.31885. Gastroenterology. 2002. PMID: 11875015
-
The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target.Curr Pharm Des. 2007;13(35):3568-75. doi: 10.2174/138161207782794167. Curr Pharm Des. 2007. PMID: 18220793 Review.
-
Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin.J Biol Chem. 2003 Jul 11;278(28):25577-84. doi: 10.1074/jbc.M301017200. Epub 2003 Apr 30. J Biol Chem. 2003. PMID: 12730231
-
Annexin II: a plasminogen-plasminogen activator co-receptor.Front Biosci. 2002 Feb 1;7:d341-8. doi: 10.2741/kim. Front Biosci. 2002. PMID: 11815288 Review.
Cited by
-
Collagen-I influences the post-translational regulation, binding partners and role of Annexin A2 in breast cancer progression.Front Oncol. 2023 Oct 24;13:1270436. doi: 10.3389/fonc.2023.1270436. eCollection 2023. Front Oncol. 2023. PMID: 37941562 Free PMC article.
-
DNA methylation and microRNA dysregulation in cancer.Mol Oncol. 2012 Dec;6(6):567-78. doi: 10.1016/j.molonc.2012.07.007. Epub 2012 Aug 10. Mol Oncol. 2012. PMID: 22902148 Free PMC article. Review.
-
Activation of the epidermal growth factor signalling pathway by tissue plasminogen activator in pancreas cancer cells.Gut. 2007 Sep;56(9):1266-74. doi: 10.1136/gut.2006.097188. Epub 2007 Apr 23. Gut. 2007. PMID: 17452424 Free PMC article.
-
Expression of annexin II and stromal tenascin C promotes epithelial to mesenchymal transition and correlates with distant metastasis in pancreatic cancer.Int J Mol Med. 2018 Aug;42(2):821-830. doi: 10.3892/ijmm.2018.3652. Epub 2018 May 2. Int J Mol Med. 2018. PMID: 29749431 Free PMC article.
-
Annexin A2: A Double-Edged Sword in Pathogen Infection.Pathogens. 2024 Jul 4;13(7):564. doi: 10.3390/pathogens13070564. Pathogens. 2024. PMID: 39057791 Free PMC article. Review.
References
-
- Lijnen HR. Elements of the fibrinolytic system. Ann N Y Acad Sci 2001;936:226–36. - PubMed
-
- Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost 2001;86:324–33. - PubMed
-
- Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 2001;21:1104–17. - PubMed
-
- Bell WR. The fibrinolytic system in neoplasia. Semin Thromb Hemost 1996;22:459–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
