Electrophysiological characterization of a recombinant human Na+-coupled nucleoside transporter (hCNT1) produced in Xenopus oocytes
- PMID: 15194733
- PMCID: PMC1665023
- DOI: 10.1113/jphysiol.2004.068189
Electrophysiological characterization of a recombinant human Na+-coupled nucleoside transporter (hCNT1) produced in Xenopus oocytes
Abstract
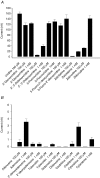
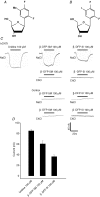
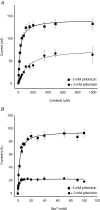
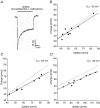
Human concentrative nucleoside transporter 1 (hCNT1) mediates active transport of nucleosides and anticancer and antiviral nucleoside drugs across cell membranes by coupling influx to the movement of Na(+) down its electrochemical gradient. The two-microelectrode voltage-clamp technique was used to measure steady-state and presteady-state currents of recombinant hCNT1 produced in Xenopus oocytes. Transport was electrogenic, phloridzin sensitive and specific for pyrimidine nucleosides and adenosine. Nucleoside analogues that induced inwardly directed Na(+) currents included the anticancer drugs 5-fluorouridine, 5-fluoro-2'-deoxyuridine, cladribine and cytarabine, the antiviral drugs zidovudine and zalcitabine, and the novel thymidine mimics 1-(2-deoxy-beta-d-ribofuranosyl)-2,4-difluoro-5-methylbenzene and 1-(2-deoxy-beta-d-ribofuranosyl)-2,4-difluoro-5-iodobenzene. Apparent K(m) values for 5-fluorouridine, 5-fluoro-2'-deoxyuridine and zidovudine were 18, 15 and 450 microm, respectively. hCNT1 was Na(+) specific, and the kinetics of steady-state uridine-evoked Na(+) currents were consistent with an ordered simultaneous transport model in which Na(+) binds first followed by uridine. Membrane potential influenced both ion binding and carrier translocation. The Na(+)-nucleoside coupling stoichiometry, determined directly by comparing the uridine-induced inward charge movement to [(14)C]uridine uptake was 1: 1. hCNT1 presteady-state currents were used to determine the fraction of the membrane field sensed by Na(+) (61%), the valency of the movable charge (-0.81) and the average number of transporters present in the oocyte plasma membrane (6.8 x 10(10) per cell). The hCNT1 turnover rate at -50 mV was 9.6 molecules of uridine transported per second.
Figures




Similar articles
-
Transport of physiological nucleosides and anti-viral and anti-neoplastic nucleoside drugs by recombinant Escherichia coli nucleoside-H(+) cotransporter (NupC) produced in Xenopus laevis oocytes.Mol Membr Biol. 2004 Jan-Feb;21(1):1-10. doi: 10.1080/0968768031000140836. Mol Membr Biol. 2004. PMID: 14668133
-
Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters.J Natl Cancer Inst. 1999 Nov 3;91(21):1876-81. doi: 10.1093/jnci/91.21.1876. J Natl Cancer Inst. 1999. PMID: 10547395
-
Electrophysiological characterization of the human Na(+)/nucleoside cotransporter 1 (hCNT1) and role of adenosine on hCNT1 function.J Biol Chem. 2004 Mar 5;279(10):8999-9007. doi: 10.1074/jbc.M311940200. Epub 2003 Dec 29. J Biol Chem. 2004. PMID: 14701834
-
Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib).Mol Membr Biol. 2001 Jan-Mar;18(1):65-72. doi: 10.1080/09687680010026313. Mol Membr Biol. 2001. PMID: 11396613 Review.
-
Recent advances in the molecular biology of nucleoside transporters of mammalian cells.Biochem Cell Biol. 1998;76(5):761-70. doi: 10.1139/bcb-76-5-761. Biochem Cell Biol. 1998. PMID: 10353709 Review.
Cited by
-
Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1).J Biol Chem. 2011 Sep 16;286(37):32552-62. doi: 10.1074/jbc.M111.236117. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795683 Free PMC article.
-
Nucleoside transporter proteins as biomarkers of drug responsiveness and drug targets.Front Pharmacol. 2015 Feb 10;6:13. doi: 10.3389/fphar.2015.00013. eCollection 2015. Front Pharmacol. 2015. PMID: 25713533 Free PMC article. Review.
-
Substituted cysteine accessibility method analysis of human concentrative nucleoside transporter hCNT3 reveals a novel discontinuous region of functional importance within the CNT family motif (G/A)XKX3NEFVA(Y/M/F).J Biol Chem. 2009 Jun 19;284(25):17281-17292. doi: 10.1074/jbc.M109.009704. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380585 Free PMC article.
-
Conserved glutamate residues Glu-343 and Glu-519 provide mechanistic insights into cation/nucleoside cotransport by human concentrative nucleoside transporter hCNT3.J Biol Chem. 2009 Jun 19;284(25):17266-17280. doi: 10.1074/jbc.M109.009613. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380587 Free PMC article.
-
Impact of intracellular domain flexibility upon properties of activated human 5-HT3 receptors.Br J Pharmacol. 2014 Apr;171(7):1617-28. doi: 10.1111/bph.12536. Br J Pharmacol. 2014. PMID: 24283776 Free PMC article.
References
-
- Acimovic Y, Coe IR. Molecular evolution of the equilibrative nucleoside transporter family: identification of novel family members in prokaryotes and eukaryotes. Mol Biol Evol. 2002;19:2199–2210. - PubMed
-
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. - PubMed
-
- Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today. 1999;5:216–224. - PubMed
-
- Bassilana M, Pourcher T, Leblanc G. Facilitated diffusion properties of melibiose permease in Escherichia coli membrane vesicles. Release of co-substrates is rate limiting for permease cycling. J Biol Chem. 1987;262:16865–16870. - PubMed
-
- Birnir B, Loo DDF, Wright EM. Voltage-clamp studies of the Na+/glucose cotransporter cloned from rabbit small intestine. Pflugers Arch. 1991;418:79–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

