An analysis of inhibitory junction potentials in the guinea-pig proximal colon
- PMID: 15194738
- PMCID: PMC1665018
- DOI: 10.1113/jphysiol.2004.065052
An analysis of inhibitory junction potentials in the guinea-pig proximal colon
Abstract
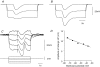
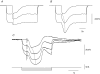
Intracellular recordings were made from either sheets or isolated bundles of the circular muscle layer of guinea-pig proximal colon and the responses evoked by stimulating inhibitory nerve fibres were analysed. Inhibitory junction potentials (IJPs), evoked by single stimuli, had two components which could be separated on their pharmacological and temporal characteristics and their voltage sensitivities. The initial component, which was abolished by apamin and reduced in amplitude by pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid (PPADS), had a brief time course: its amplitude was changed when the external concentration of potassium ions ([K+](o)) was changed. The second component of the IJP had a slower onset than the first component, was abolished by l-nitroarginine (NOLA) and oxadiazolo quinoxalin-1-one (ODQ), an inhibitor of soluble guanylate cyclase: its amplitude was little affected by changing [K+](o) and was increased when the membrane potential of the circular layer was hyperpolarized. The observations suggest that the initial component of the IJP results from the release of ATP which triggers an increase in membrane conductance to K+ and that the second component results from the release of nitric oxide which suppresses a background inward current.
Figures







Similar articles
-
Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon.J Auton Nerv Syst. 1998 Apr 30;69(2-3):115-21. doi: 10.1016/s0165-1838(98)00012-5. J Auton Nerv Syst. 1998. PMID: 9696266
-
Interaction between excitatory and inhibitory metabotropic pathways in the guinea-pig antrum.J Physiol. 2003 Jul 1;550(Pt 1):181-9. doi: 10.1113/jphysiol.2003.043273. J Physiol. 2003. PMID: 12879868 Free PMC article.
-
Characterization of the membrane conductance changes underlying the apamin-resistant NANC inhibitory junction potential in the guinea-pig proximal and distal colon.J Auton Nerv Syst. 1996 Aug 27;60(1-2):31-42. doi: 10.1016/0165-1838(96)00024-0. J Auton Nerv Syst. 1996. PMID: 8884693
-
Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1483-9. doi: 10.1152/ajpgi.00450.2006. Epub 2007 Feb 22. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17322065
-
Effects of nitric oxide donors, S-nitroso-L-cysteine and sodium nitroprusside, on the whole-cell and single channel currents in single myocytes of the guinea-pig proximal colon.Br J Pharmacol. 1998 Feb;123(3):505-17. doi: 10.1038/sj.bjp.0701605. Br J Pharmacol. 1998. PMID: 9504392 Free PMC article.
Cited by
-
Purinergic mechanisms in the control of gastrointestinal motility.Purinergic Signal. 2008 Sep;4(3):197-212. doi: 10.1007/s11302-007-9081-z. Epub 2007 Oct 6. Purinergic Signal. 2008. PMID: 18368521 Free PMC article.
-
Comparison of inhibitory neuromuscular transmission in the Cynomolgus monkey IAS and rectum: special emphasis on differences in purinergic transmission.J Physiol. 2018 Nov;596(22):5319-5341. doi: 10.1113/JP275437. Epub 2018 Oct 13. J Physiol. 2018. PMID: 30198065 Free PMC article.
-
Stretch-dependent sensitization of post-junctional neural effectors in colonic muscles.Neurogastroenterol Motil. 2013 Feb;25(2):e101-13. doi: 10.1111/nmo.12059. Epub 2013 Jan 2. Neurogastroenterol Motil. 2013. PMID: 23279087 Free PMC article.
-
Role of K(+) channels in the regulation of electrical spontaneous activity of the mouse small intestine.Pflugers Arch. 2007 Dec;455(3):505-14. doi: 10.1007/s00424-007-0306-3. Epub 2007 Jun 30. Pflugers Arch. 2007. PMID: 17602242
-
The purinergic neurotransmitter revisited: a single substance or multiple players?Pharmacol Ther. 2014 Nov;144(2):162-91. doi: 10.1016/j.pharmthera.2014.05.012. Epub 2014 Jun 2. Pharmacol Ther. 2014. PMID: 24887688 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

