On the fate of skeletal myoblasts in a cardiac environment: down-regulation of voltage-gated ion channels
- PMID: 15194742
- PMCID: PMC1665019
- DOI: 10.1113/jphysiol.2003.060186
On the fate of skeletal myoblasts in a cardiac environment: down-regulation of voltage-gated ion channels
Abstract
We have analysed the voltage-gated ion channels and fusion competence of skeletal muscle myoblasts labelled with green fluorescent protein (GFP) and the membrane dye PKH transplanted into the infarcted myocardium of syngenic rats. After cell transplantation the animals were killed and GFP(+)-PKH(+) myoblasts enzymatically isolated for subsequent studies of ionic currents through voltage-gated sodium, calcium and potassium channels. A down-regulation of all three types of ion channels after engraftment was observed. The fraction of cells with calcium (68%) and sodium channels (65%) declined to zero within 24 h and 1 week, respectively. Down-regulation of potassium currents (90% in control) occurred within 2 weeks to about 30%. Before injection myoblasts expressed predominantly transient outward potassium channels whereas after isolation from the myocardium exclusively rapid delayed rectifier channels. The currents recovered completely between 1 and 6 weeks under cell culture conditions. The down-regulation of ion channels and changes in potassium current kinetics suggest that the environment provided by infarcted myocardium affects expression of voltage-gated ion channels of skeletal myoblasts.
Figures
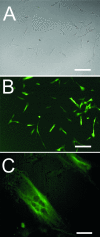

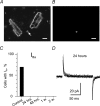


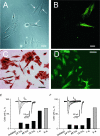
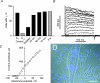

Similar articles
-
Exogenous connexin43-expressing autologous skeletal myoblasts ameliorate mechanical function and electrical activity of the rabbit heart after experimental infarction.Int J Exp Pathol. 2015 Feb;96(1):42-53. doi: 10.1111/iep.12109. Epub 2014 Dec 22. Int J Exp Pathol. 2015. PMID: 25529770 Free PMC article.
-
Differential effects of crambescins and crambescidin 816 in voltage-gated sodium, potassium and calcium channels in neurons.Chem Res Toxicol. 2013 Jan 18;26(1):169-78. doi: 10.1021/tx3004483. Epub 2013 Jan 9. Chem Res Toxicol. 2013. PMID: 23270282
-
Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium.Cardiovasc Res. 2005 Mar 1;65(4):851-60. doi: 10.1016/j.cardiores.2004.11.022. Cardiovasc Res. 2005. PMID: 15721865
-
[Recent progress of research on cardiac ion channels].Nihon Rinsho. 1993 May;51(5):1140-5. Nihon Rinsho. 1993. PMID: 8392633 Review. Japanese.
-
Localization of voltage-gated ion channels in mammalian brain.Annu Rev Physiol. 2004;66:477-519. doi: 10.1146/annurev.physiol.66.032102.113328. Annu Rev Physiol. 2004. PMID: 14977411 Review.
Cited by
-
Exogenous connexin43-expressing autologous skeletal myoblasts ameliorate mechanical function and electrical activity of the rabbit heart after experimental infarction.Int J Exp Pathol. 2015 Feb;96(1):42-53. doi: 10.1111/iep.12109. Epub 2014 Dec 22. Int J Exp Pathol. 2015. PMID: 25529770 Free PMC article.
-
Repairing damaged myocardium: evaluating cells used for cardiac regeneration.Curr Treat Options Cardiovasc Med. 2008 Feb;10(1):59-72. doi: 10.1007/s11936-008-0007-z. Curr Treat Options Cardiovasc Med. 2008. PMID: 18325308
-
Restoring heart function and electrical integrity: closing the circuit.NPJ Regen Med. 2017 Apr 7;2:9. doi: 10.1038/s41536-017-0015-2. eCollection 2017. NPJ Regen Med. 2017. PMID: 29302345 Free PMC article. Review.
References
-
- Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat ML, Gabbiani G, Bader CR. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. - PubMed
-
- Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. - PubMed
-
- Berridge MJ, Bootman MD, Lipp P. Calcium: a life and death signal. Nature. 1998;395:645–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

