PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways
- PMID: 15194744
- PMCID: PMC1665017
- DOI: 10.1113/jphysiol.2004.061721
PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways
Abstract
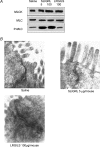
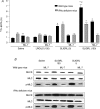
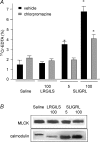
Activation of colonic proteinase-activated receptor-2 (PAR(2)) caused inflammation and increased mucosal permeability in mouse colon. The present study was aimed at characterizing the possible links between these two phenomena. We evaluated the effects of intracolonic infusion of PAR(2)-activating peptide, SLIGRL, on colonic paracellular permeability and inflammation at two different doses, 5 and 100 microg per mouse, in an attempt to discriminate between both PAR(2)-mediated effects. We further investigated the possible involvement of interferon gamma (IFN-gamma) and calmodulin-dependent activation of myosin light chain kinase (MLCK), and alterations of zonula occludens-1 (ZO-1) localization in PAR(2)-induced responses. Thus, at the lower dose, SLIGRL increased colonic permeability without causing inflammation. Western blotting showed phosphorylation of mucosal myosin light chain (MLC) expression after both doses of SLIGRL. Moreover, while the MLCK inhibitor, ML-7, abolished the permeability effects of the low dose of SLIGRL, it only partially inhibited that of the high dose. In IFN-gamma-deficient mice (B6 ifng(-/-)), the increases in permeability were similar for both doses of SLIGRL and prevented by ML-7. In addition, MLCK immunoprecipitation revealed an increase of calmodulin binding to MLCK in the mucosa of mice treated with either dose of SLIGRL. Finally, we have shown that direct activation of PAR(2) on enterocytes is responsible for increased permeability and ZO-1 disruption. Moreover, SLIGRL at a dose that does not produce inflammation increases permeability via calmodulin activation, which binds and activates MLCK. The resulting tight junction opening does not depend upon IFN-gamma secretion, while the increased permeability in response to the high dose of PAR(2) agonist involves IFN-gamma secretion.
Figures
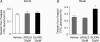

Similar articles
-
Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability.J Immunol. 2003 Apr 15;170(8):4296-300. doi: 10.4049/jimmunol.170.8.4296. J Immunol. 2003. PMID: 12682265
-
Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction.Gut. 2006 May;55(5):655-61. doi: 10.1136/gut.2005.078675. Epub 2005 Nov 18. Gut. 2006. PMID: 16299034 Free PMC article.
-
Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity.Gut. 2008 May;57(5):591-9. doi: 10.1136/gut.2007.140210. Epub 2008 Jan 14. Gut. 2008. PMID: 18194983
-
Protective role for protease-activated receptor-2 against influenza virus pathogenesis via an IFN-gamma-dependent pathway.J Immunol. 2009 Jun 15;182(12):7795-802. doi: 10.4049/jimmunol.0803743. J Immunol. 2009. PMID: 19494303
-
Protease activated receptor 2: a new target for IBS treatment.Eur Rev Med Pharmacol Sci. 2008 Aug;12 Suppl 1:95-102. Eur Rev Med Pharmacol Sci. 2008. PMID: 18924448 Review.
Cited by
-
The expression of protease-activated receptor 2 and 4 in the colon of irritable bowel syndrome patients.Dig Dis Sci. 2012 Jan;57(1):58-64. doi: 10.1007/s10620-011-1827-3. Epub 2011 Jul 29. Dig Dis Sci. 2012. PMID: 21800160
-
Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability.J Immunol. 2008 Apr 15;180(8):5653-61. doi: 10.4049/jimmunol.180.8.5653. J Immunol. 2008. PMID: 18390750 Free PMC article.
-
Role of Stress on Driving the Intestinal Paracellular Permeability.Curr Issues Mol Biol. 2023 Nov 18;45(11):9284-9305. doi: 10.3390/cimb45110581. Curr Issues Mol Biol. 2023. PMID: 37998758 Free PMC article. Review.
-
Dexamethasone prevents visceral hyperalgesia but not colonic permeability increase induced by luminal protease-activated receptor-2 agonist in rats.Gut. 2007 Aug;56(8):1072-8. doi: 10.1136/gut.2006.115352. Epub 2007 Feb 19. Gut. 2007. PMID: 17309885 Free PMC article.
-
Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase.Dig Dis Sci. 2010 Oct;55(10):2922-8. doi: 10.1007/s10620-009-1094-8. Epub 2010 Jan 20. Dig Dis Sci. 2010. PMID: 20087660
References
-
- Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. - PubMed
-
- Berger P, Tunon-De-Lara JM, Savineau JP, Marthan R. Selected contribution: tryptase-induced PAR-2-mediated Ca2+ signaling in human airway smooth muscle cells. J Appl Physiol. 2001;91:995–1003. - PubMed
-
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

