Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages
- PMID: 15194768
- PMCID: PMC421670
- DOI: 10.1128/JVI.78.13.6915-6926.2004
Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages
Erratum in
- J Virol. 2005 Mar;79(5):3227
Abstract
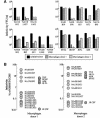
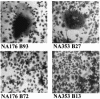
Complete envelope genes were amplified from autopsy brain tissue of five individuals who had died of AIDS and had neurological complications. Lymph node samples were included for two of the patients. Nineteen different envelope clones from the five patients had distinct V1V2 sequences. Thirteen of the envelopes were functional and conferred fusigenicity and infectivity for CD4(+) CCR5(+) cells. Infectivity and cell-cell fusion assays showed that most envelopes used both CCR5 and CCR3. One brain-derived envelope used a broad range of coreceptors, while three other brain envelopes from one individual were restricted to CCR5. However, there was no correlation between tissue of origin and coreceptor use. Envelopes showed two very distinct phenotypes depending on their capacity to infect macrophages and to exploit low levels of CD4 and/or CCR5 for infection. Envelopes that were highly fusigenic and tropic for macrophages were identified in brain tissue from four of the five patients. The enhanced macrophage tropism correlated with reduced sensitivity to inhibition by Q4120, a CD4-specific antibody, but not with sensitivity to the CCR5 inhibitor, TAK779. The highly macrophage-tropic envelopes were able to infect cells expressing low levels of CD4 and/or CCR5. Comparison with several well-characterized macrophage-tropic envelopes showed that the four identified patient envelopes were at the top limit of macrophage tropism. In contrast, all four lymph node-derived envelopes exhibited a non-macrophage-tropic phenotype and required high levels of CD4 for infection. Our data support the presence of envelopes that are highly fusigenic and tropic for macrophages in the brains of patients with neurological complications. These envelopes are able to infect cells that express low levels of CD4 and/or CCR5 and may have adapted for replication in brain macrophages and microglia, which are known to express limited amounts of CD4.
Figures



References
-
- Berger, E. A., R. W. Doms, E.-M. Fenyo, B. T. M. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240. - PubMed
-
- Chesebro, B., K. Wehrly, J. Nishio, and S. Perryman. 1992. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J. Virol. 66:6547-6554. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

