Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates
- PMID: 15194776
- PMCID: PMC421664
- DOI: 10.1128/JVI.78.13.6995-7003.2004
Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates
Abstract
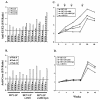
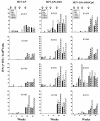
We have previously reported the production of hepatitis C virus-like particles (HCV-LP) using a recombinant baculovirus containing the cDNA of the HCV structural proteins (core, E1, and E2). These particles resemble the putative HCV virions and are capable of inducing strong and broad humoral and cellular immune responses in mice. Here we present evidence on the immunogenicity of HCV-LP and the effects of novel adjuvant systems in a nonhuman primate model, the baboon. Three groups of four baboons were immunized with HCV-LP, HCV-LP and adjuvant AS01B (monophosphoryl lipid A and QS21), or HCV-LP and the combination of AS01B and CpG oligodeoxynucleotides 10105. After four immunizations over an 8-month period, all animals developed HCV-specific humoral and cellular immune responses including antibodies to HCV structural proteins and gamma interferon(+) (IFN-gamma(+))CD4(+) and IFN-gamma(+)CD8(+) T-cell responses. The immunogenicity of HCV-LP was only marginally enhanced by the use of adjuvants. The overall HCV-specific immune responses were broad and long lasting. Our results suggest that HCV-LP is a potent immunogen to induce HCV-specific humoral and cellular immune responses in primates and may be a promising approach to develop novel preventive and therapeutic modalities.
Figures


Similar articles
-
Hepatitis C virus-like particles combined with novel adjuvant systems enhance virus-specific immune responses.Hepatology. 2003 Jan;37(1):52-9. doi: 10.1053/jhep.2003.50000. Hepatology. 2003. PMID: 12500188
-
Vigorous hepatitis C virus-specific CD4+ and CD8+ T cell responses induced by protein immunization in the presence of Montanide ISA720 plus synthetic oligodeoxynucleotides containing immunostimulatory cytosine-guanine dinucleotide motifs.J Infect Dis. 2006 Feb 15;193(4):563-72. doi: 10.1086/499823. Epub 2006 Jan 11. J Infect Dis. 2006. PMID: 16425136
-
CpG immuno-stimulatory motifs enhance humoral immune responses against hepatitis C virus core protein after DNA-based immunization.Arch Virol. 2003 Mar;148(3):435-48. doi: 10.1007/s00705-002-0935-y. Arch Virol. 2003. PMID: 12607097
-
[Hepatitis C immunology].Rev Invest Clin. 1999 Sep-Oct;51(5):315-22. Rev Invest Clin. 1999. PMID: 10614142 Review. Spanish.
-
Hepatitis C vaccines: Inducing and challenging memory T cells.Hepatology. 2006 Jun;43(6):1395-8. doi: 10.1002/hep.21210. Hepatology. 2006. PMID: 16729320 Review.
Cited by
-
Prospects for prophylactic hepatitis C vaccines based on virus-like particles.Hum Vaccin Immunother. 2013 May;9(5):1112-8. doi: 10.4161/hv.23900. Epub 2013 Feb 13. Hum Vaccin Immunother. 2013. PMID: 23406827 Free PMC article. Review.
-
Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011 Mar-Apr;3(2):174-196. doi: 10.1002/wnan.119. Epub 2010 Sep 24. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011. PMID: 20872839 Free PMC article. Review.
-
Baculovirus as versatile vectors for protein expression in insect and mammalian cells.Nat Biotechnol. 2005 May;23(5):567-75. doi: 10.1038/nbt1095. Nat Biotechnol. 2005. PMID: 15877075 Free PMC article. Review.
-
Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus.J Virol. 2008 Nov;82(21):10896-905. doi: 10.1128/JVI.01179-08. Epub 2008 Aug 27. J Virol. 2008. PMID: 18753204 Free PMC article.
-
Virus-like particle vaccine containing hemagglutinin confers protection against 2009 H1N1 pandemic influenza.Clin Vaccine Immunol. 2011 Dec;18(12):2010-7. doi: 10.1128/CVI.05206-11. Epub 2011 Oct 26. Clin Vaccine Immunol. 2011. PMID: 22030367 Free PMC article.
References
-
- Ambrosch, F., G. Wiedermann, M. Kundi, G. Leroux-Roels, I. Desombere, N. Garcon, C. Thiriart, M. Slaoui, and S. Thoelen. 2000. A hepatitis B vaccine formulated with a novel adjuvant system. Vaccine 18:2095-2101. - PubMed
-
- Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. - PubMed
-
- Baumert, T. F., J. Vergalla, J. Satoi, M. Thomson, M. Lechmann, D. Herion, H. B. Greenberg, S. Ito, and T. J. Liang. 1999. Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology 117:1397-1407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

