Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion
- PMID: 15194790
- PMCID: PMC421652
- DOI: 10.1128/JVI.78.13.7138-7147.2004
Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion
Abstract
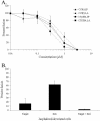
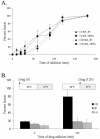
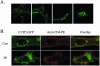
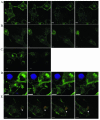
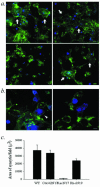
The membrane fusion events which initiate human immunodeficiency virus type 1 (HIV-1) infection and promote cytopathic syncytium formation in infected cells commence with the binding of the HIV envelope glycoprotein (Env) to CD4 and an appropriate coreceptor. Here, we show that HIV Env-coreceptor interactions activate Rac-1 GTPase and stimulate the actin filament network reorganizations that are requisite components of the cell fusion process. Disrupting actin filament dynamics with jasplakinolide or latrunculin A arrested fusion at a late step in the formation of Env-CD4-coreceptor complexes. Time-lapse confocal microscopy of living cells revealed vigorous activity of actin-based, target cell membrane extensions at the target cell-Env-expressing cell interface. The expression of dominant-negative forms of actin-regulating Rho-family GTPases established that HIV Env-mediated syncytium formation relies on Rac-1 but not on Cdc42 or Rho activation in target cells. Similar dependencies were found when cell fusion was induced by Env expressed on viral or cellular membranes. Additionally, Rac activity was specifically upregulated in a coreceptor-dependent manner in fusion reaction cell lysates. These results define a role for HIV Env-coreceptor interactions in activating the cellular factors essential for virus-cell and cell-cell fusion and provide evidence for the participation of pertussis toxin-insensitive signaling pathways in HIV-induced membrane fusion.
Figures

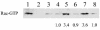

References
-
- Alkhatib, G., M. Locati, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. - PubMed
-
- Amara, A., A. Vidy, G. Boulla, K. Mollier, J. Garcia-Perez, J. Alcami, C. Blanpain, M. Parmentier, J.-L. Virelizier, P. Charneau, and F. Arenzana-Seisdedos. 2003. G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates. J. Virol. 77:2550-2558. - PMC - PubMed
-
- Biscone, M. J., T. C. Pierson, and R. W. Doms. 2002. Opportunities and challenges in targeting HIV entry. Curr. Opin. Pharmacol. 2:529-533. - PubMed
-
- Bubb, M. R., I. Spector, B. B. Beyer, and K. M. Fosen. 2000. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J. Biol. Chem. 275:5163-5170. - PubMed
-
- Cicala, C., J. Arthos, M. Ruiz, M. Vaccarezza, A. Rubbert, A. Riva, K. Wildt, O. Cohen, and A. S. Fauci. 1999. Induction of phosphorylation and intracellular association of CC chemokine receptor 5 and focal adhesion kinase in primary human CD4+ T cells by macrophage-tropic HIV envelope. J. Immunol. 163:420-426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

