Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses
- PMID: 15194792
- PMCID: PMC421692
- DOI: 10.1128/JVI.78.13.7153-7164.2004
Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses
Abstract
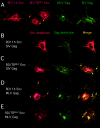
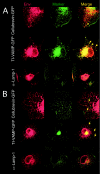
Glycoproteins derived from most retroviruses and from several families of enveloped viruses can form infectious pseudotypes with murine leukemia virus (MLV) and lentiviral core particles, like the MLV envelope glycoproteins (Env) that are incorporated on either virus type. However, coexpression of a given glycoprotein with heterologous core proteins does not always give rise to highly infectious viral particles, and restrictions on pseudotype formation have been reported. To understand the mechanisms that control the recruitment of viral surface glycoproteins on lentiviral and retroviral cores, we exploited the fact that the feline endogenous retrovirus RD114 glycoprotein does not efficiently pseudotype lentiviral cores derived from simian immunodeficiency virus, whereas it is readily incorporated onto MLV particles. Our results indicate that recruitment of glycoproteins by the MLV and lentiviral core proteins occurs in intracellular compartments and not at the cell surface. We found that Env and core protein colocalization in intracytoplasmic vesicles is required for pseudotype formation. By investigating MLV/RD114 Env chimeras, we show that signals in the cytoplasmic tail of either glycoprotein differentially influenced their intracellular localization; that of MLV allows endosomal localization and hence recruitment by both lentiviral and MLV cores. Furthermore, we found that upon membrane binding, MLV core proteins could relocalize Env glycoproteins in late endosomes and allow their incorporation on viral particles. Thus, intracellular colocalization, as well as interactions between Env and core proteins, may influence the recruitment of the glycoprotein onto viral particles and generate infectious pseudotyped viruses.
Figures


Similar articles
-
An acidic cluster of the cytoplasmic tail of the RD114 virus glycoprotein controls assembly of retroviral envelopes.Traffic. 2007 Jul;8(7):835-47. doi: 10.1111/j.1600-0854.2007.00581.x. Epub 2007 Jun 4. Traffic. 2007. PMID: 17547695
-
Intracellular versus cell surface assembly of retroviral pseudotypes is determined by the cellular localization of the viral glycoprotein, its capacity to interact with Gag, and the expression of the Nef protein.J Biol Chem. 2006 Jan 6;281(1):528-42. doi: 10.1074/jbc.M506070200. Epub 2005 Sep 28. J Biol Chem. 2006. PMID: 16195228
-
Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1.Virology. 2000 Jul 20;273(1):16-20. doi: 10.1006/viro.2000.0394. Virology. 2000. PMID: 10891403
-
Mechanisms for Env glycoprotein acquisition by retroviruses.AIDS Res Hum Retroviruses. 2011 Mar;27(3):239-47. doi: 10.1089/AID.2010.0350. Epub 2011 Feb 22. AIDS Res Hum Retroviruses. 2011. PMID: 21247353 Free PMC article. Review.
-
Endosomes, exosomes and Trojan viruses.Trends Microbiol. 2004 Jul;12(7):310-6. doi: 10.1016/j.tim.2004.05.004. Trends Microbiol. 2004. PMID: 15223058 Review.
Cited by
-
Codon Optimization Leads to Functional Impairment of RD114-TR Envelope Glycoprotein.Mol Ther Methods Clin Dev. 2017 Jan 11;4:102-114. doi: 10.1016/j.omtm.2017.01.002. eCollection 2017 Mar 17. Mol Ther Methods Clin Dev. 2017. PMID: 28344996 Free PMC article.
-
Multiple Gag domains contribute to selective recruitment of murine leukemia virus (MLV) Env to MLV virions.J Virol. 2013 Feb;87(3):1518-27. doi: 10.1128/JVI.02604-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152533 Free PMC article.
-
HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane.PLoS Pathog. 2006 May;2(5):e39. doi: 10.1371/journal.ppat.0020039. Epub 2006 May 12. PLoS Pathog. 2006. PMID: 16699598 Free PMC article.
-
Amphotropic murine leukaemia virus envelope protein is associated with cholesterol-rich microdomains.Virol J. 2005 Apr 19;2:36. doi: 10.1186/1743-422X-2-36. Virol J. 2005. PMID: 15840168 Free PMC article.
-
A human endogenous retrovirus-derived gene that can contribute to oncogenesis by activating the ERK pathway and inducing migration and invasion.PLoS Pathog. 2017 Jun 26;13(6):e1006451. doi: 10.1371/journal.ppat.1006451. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28651004 Free PMC article.
References
-
- Alberts, P., and T. Galli. 2003. The cell outgrowth secretory endosome (COSE): a specialized compartment involved in neuronal morphogenesis. Biol. Cell 95:419-424. - PubMed
-
- Basyuk, E., T. Galli, M. Mougel, J. M. Blanchard, M. Sitbon, and E. Bertrand. 2003. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell 5:161-174. - PubMed
-
- Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

