Modulation of p53 cellular function and cell death by African swine fever virus
- PMID: 15194793
- PMCID: PMC421689
- DOI: 10.1128/JVI.78.13.7165-7174.2004
Modulation of p53 cellular function and cell death by African swine fever virus
Abstract
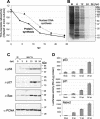
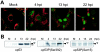
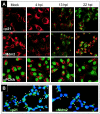
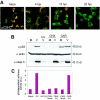
Modulation of the activity of tumor suppressor p53 is a key event in the replication of many viruses. We have studied the function of p53 in African swine fever virus (ASFV) infection by determining the expression and activity of this transcription factor in infected cells. p53 levels are increased at early times of infection and are maintained throughout the infectious cycle. The protein is transcriptionally active, stabilized by phosphorylation, and localized in the nucleus. p53 induces the expression of p21 and Mdm2. Strikingly, these two proteins are located at the cytoplasmic virus factories. The retention of Mdm2 at the factory may represent a viral mechanism to prevent p53 inactivation by the protein. The expression of apoptotic proteins, such as Bax or active caspase-3, is also increased following ASFV infection, although the increase in caspase-3 does not appear to be, at least exclusively, p53 dependent. Bax probably plays a role in the induction of apoptosis in the infected cells, as suggested by the release of cytochrome c from the mitochondria. The significance of p21 induction and localization is discussed in relation to the shutoff of cellular DNA synthesis that is observed in ASFV-infected cells.
Figures
Similar articles
-
p53 expression in CMV-infected cells: association with the alternative expression of the p53 transactivated genes p21/WAF1 and MDM2.Histopathology. 1997 Feb;30(2):120-5. doi: 10.1046/j.1365-2559.1997.d01-577.x. Histopathology. 1997. PMID: 9067734
-
Combretastatin-A4 prodrug induces mitotic catastrophe in chronic lymphocytic leukemia cell line independent of caspase activation and poly(ADP-ribose) polymerase cleavage.Clin Cancer Res. 2002 Aug;8(8):2735-41. Clin Cancer Res. 2002. PMID: 12171907
-
Early intranuclear replication of African swine fever virus genome modifies the landscape of the host cell nucleus.Virus Res. 2015 Dec 2;210:1-7. doi: 10.1016/j.virusres.2015.07.006. Epub 2015 Jul 13. Virus Res. 2015. PMID: 26183880
-
P21/waf1 protein expression in nasopharyngeal carcinoma. Comparative study with PCNA, p53 and MDM-2 protein expression.Anticancer Res. 1997 Jul-Aug;17(4A):2615-9. Anticancer Res. 1997. PMID: 9252690
-
The Wheel of p53 Helps to Drive the Immune System.Int J Mol Sci. 2023 Apr 21;24(8):7645. doi: 10.3390/ijms24087645. Int J Mol Sci. 2023. PMID: 37108808 Free PMC article. Review.
Cited by
-
A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication.Adv Virus Res. 2007;70:101-82. doi: 10.1016/S0065-3527(07)70004-0. Adv Virus Res. 2007. PMID: 17765705 Free PMC article. Review.
-
Identification of differentially activated cell-signaling networks associated with pichinde virus pathogenesis by using systems kinomics.J Virol. 2007 Feb;81(4):1923-33. doi: 10.1128/JVI.02199-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151108 Free PMC article.
-
p53 and miR-34a Feedback Promotes Lung Epithelial Injury and Pulmonary Fibrosis.Am J Pathol. 2017 May;187(5):1016-1034. doi: 10.1016/j.ajpath.2016.12.020. Epub 2017 Mar 6. Am J Pathol. 2017. PMID: 28273432 Free PMC article.
-
Phenotyping and susceptibility of established porcine cells lines to African Swine Fever Virus infection and viral production.Sci Rep. 2017 Sep 4;7(1):10369. doi: 10.1038/s41598-017-09948-x. Sci Rep. 2017. PMID: 28871180 Free PMC article.
-
African swine fever virus-cell interactions: from virus entry to cell survival.Virus Res. 2013 Apr;173(1):42-57. doi: 10.1016/j.virusres.2012.12.006. Epub 2012 Dec 20. Virus Res. 2013. PMID: 23262167 Free PMC article. Review.
References
-
- Agarwal, M. L., W. R. Taylor, M. V. Chernov, O. B. Chernova, and G. R. Stark. 1998. The p53 network. J. Biol. Chem. 273:1-4. - PubMed
-
- Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. - PubMed
-
- Appella, E., and C. W. Anderson. 2000. Signaling to p53: breaking the posttranslational modification code. Pathol. Biol. (Paris) 48:227-245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

