Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection
- PMID: 15194794
- PMCID: PMC421678
- DOI: 10.1128/JVI.78.13.7175-7185.2004
Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection
Abstract
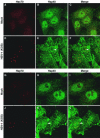
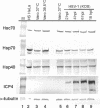
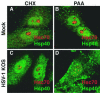
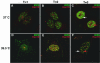
Herpes simplex virus type 1 (HSV-1) encodes a portal protein that forms a large oligomeric structure believed to provide the conduit for DNA entry and exit from the capsid. Chaperone proteins often facilitate the folding and multimerization of such complex structures. In this report, we show that cellular chaperone proteins, components of the 26S proteasome, and ubiquitin-conjugated proteins are sequestered in discrete foci in the nucleus of the infected cell. The immediate-early viral protein ICP0 was shown to be necessary to establish these foci at early times during infection and sufficient to redistribute chaperone molecules in transfected cells. Furthermore, we found that not only is the portal protein, UL6, localized to these sites during infection, but it is also a substrate for ubiquitin modification. Our results suggest that HSV-1 has evolved an elegant mechanism for facilitating protein quality control at specialized foci within the nucleus.
Figures
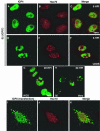
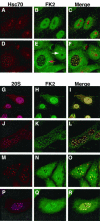
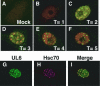
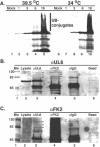
Similar articles
-
The Herpes Simplex Virus 1 Immediate Early Protein ICP22 Is a Functional Mimic of a Cellular J Protein.J Virol. 2020 Jan 31;94(4):e01564-19. doi: 10.1128/JVI.01564-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31748398 Free PMC article.
-
A pre-immediate-early role for tegument ICP0 in the proteasome-dependent entry of herpes simplex virus.J Virol. 2011 Jun;85(12):5910-8. doi: 10.1128/JVI.00267-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471243 Free PMC article.
-
The herpes simplex virus type 1 transactivator ICPO mediates aberrant intracellular localization of the viral helicase/primase complex subunits.Virology. 1996 Jun 15;220(2):495-501. doi: 10.1006/viro.1996.0338. Virology. 1996. PMID: 8669135
-
Herpes simplex virus type 2 encodes a heat shock protein homologue with apoptosis regulatory functions.Front Biosci. 2005 Sep 1;10:2788-803. doi: 10.2741/1736. Front Biosci. 2005. PMID: 15970534 Review.
-
A surprising role for the proteasome in the regulation of herpesvirus infection.Trends Biochem Sci. 1999 Aug;24(8):293-5. doi: 10.1016/s0968-0004(99)01433-4. Trends Biochem Sci. 1999. PMID: 10431170 Review. No abstract available.
Cited by
-
An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses.PLoS Pathog. 2015 Aug 5;11(8):e1005082. doi: 10.1371/journal.ppat.1005082. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26244496 Free PMC article.
-
FEZ1 phosphorylation regulates HSPA8 localization and interferon-stimulated gene expression.Cell Rep. 2022 Feb 15;38(7):110396. doi: 10.1016/j.celrep.2022.110396. Cell Rep. 2022. PMID: 35172151 Free PMC article.
-
Synergistic Effects of Bortezomib-OV Therapy and Anti-Invasive Strategies in Glioblastoma: A Mathematical Model.Cancers (Basel). 2019 Feb 13;11(2):215. doi: 10.3390/cancers11020215. Cancers (Basel). 2019. PMID: 30781871 Free PMC article.
-
Nuclear pore composition and gating in herpes simplex virus-infected cells.J Virol. 2008 Sep;82(17):8392-9. doi: 10.1128/JVI.00951-08. Epub 2008 Jun 18. J Virol. 2008. PMID: 18562518 Free PMC article.
-
MG-132 reduces virus release in Bovine herpesvirus-1 infection.Sci Rep. 2017 Oct 17;7(1):13306. doi: 10.1038/s41598-017-13717-1. Sci Rep. 2017. PMID: 29042667 Free PMC article.
References
-
- Besse, S., and F. Puvion-Dutilleul. 1996. Distribution of ribosomal genes in nucleoli of herpes simplex virus type 1 infected cells. Eur. J. Cell Biol. 71:33-44. - PubMed
-
- Black, L. W. 1989. DNA packaging in dsDNA bacteriophages. Annu. Rev. Microbiol. 43:267-292. - PubMed
-
- Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

