Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection
- PMID: 15194802
- PMCID: PMC421679
- DOI: 10.1128/JVI.78.13.7264-7269.2004
Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection
Abstract
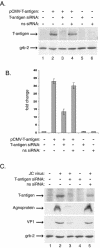
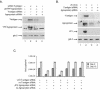
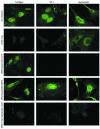
The human polyomavirus, JC virus (JCV), encodes two regulatory proteins at the early (T antigen) and the late (agnoprotein) phases of viral infection whose activities are important for the production of the viral capsid proteins and the dysregulation of several host factors and their functions. For this study, we designed and utilized an RNA interference strategy via small interfering RNAs (siRNAs) that targeted the expression of T antigen and agnoprotein in human astrocytic cells. The treatment of cells with specific siRNA oligonucleotides targeting a conserved region of T antigen, nucleotides (nt) 4256 to 4276 (Mad-1 strain), caused a >50% decline in the level of T antigen and in its transcriptional activity upon the viral capsid genes as well as a significant reduction in viral DNA replication in infected cells. Similarly, a single siRNA that aimed at nt 324 to 342 of agnoprotein noticeably reduced early and late viral protein production. A combined treatment of the infected cells with both T-antigen and agnoprotein siRNAs completely abolished viral capsid protein production, indicative of the ability of the siRNAs to effectively halt multiplication of the virus in infected cells. These observations provide a new avenue for possible treatments of patients with the JCV-induced demyelinating disease progressive multifocal leukoencephalopathy.
Figures
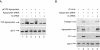
Similar articles
-
Cross-interaction between JC virus agnoprotein and human immunodeficiency virus type 1 (HIV-1) Tat modulates transcription of the HIV-1 long terminal repeat in glial cells.J Virol. 2006 Sep;80(18):9288-99. doi: 10.1128/JVI.02138-05. J Virol. 2006. PMID: 16940540 Free PMC article.
-
Inhibition of virus production in JC virus-infected cells by postinfection RNA interference.J Virol. 2004 Jul;78(13):7270-3. doi: 10.1128/JVI.78.13.7270-7273.2004. J Virol. 2004. PMID: 15194803 Free PMC article.
-
Expression of JC virus agnoprotein in progressive multifocal leukoencephalopathy brain.Acta Neuropathol. 2002 Aug;104(2):130-6. doi: 10.1007/s00401-002-0526-8. Epub 2002 May 24. Acta Neuropathol. 2002. PMID: 12111355
-
[Recent research on the JC virus].Brain Nerve. 2007 Feb;59(2):101-8. Brain Nerve. 2007. PMID: 17380774 Review. Japanese.
-
[Recent research on the JC virus].No To Shinkei. 2007 Feb;59(2):101-8. No To Shinkei. 2007. PMID: 17315751 Review. Japanese.
Cited by
-
Cross-interaction between JC virus agnoprotein and human immunodeficiency virus type 1 (HIV-1) Tat modulates transcription of the HIV-1 long terminal repeat in glial cells.J Virol. 2006 Sep;80(18):9288-99. doi: 10.1128/JVI.02138-05. J Virol. 2006. PMID: 16940540 Free PMC article.
-
Evidence for modulation of BAG3 by polyomavirus JC early protein.J Gen Virol. 2009 Jul;90(Pt 7):1629-1640. doi: 10.1099/vir.0.008722-0. Epub 2009 Mar 12. J Gen Virol. 2009. PMID: 19282432 Free PMC article.
-
RNA interference-mediated virus clearance from cells both acutely and chronically infected with the prototypic arenavirus lymphocytic choriomeningitis virus.J Virol. 2005 Sep;79(17):11071-81. doi: 10.1128/JVI.79.17.11071-11081.2005. J Virol. 2005. PMID: 16103158 Free PMC article.
-
Broad-spectrum antiviral activity of small interfering RNA targeting the conserved RNA termini of Lassa virus.Antimicrob Agents Chemother. 2007 Jun;51(6):2215-8. doi: 10.1128/AAC.01368-06. Epub 2007 Mar 19. Antimicrob Agents Chemother. 2007. PMID: 17371814 Free PMC article.
-
A review on current status of antiviral siRNA.Rev Med Virol. 2018 Jul;28(4):e1976. doi: 10.1002/rmv.1976. Epub 2018 Apr 15. Rev Med Virol. 2018. PMID: 29656441 Free PMC article. Review.
References
-
- Aksamit, A. 2001. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J. Neurovirol. 7:386-390. - PubMed
-
- Berger, J. R., and M. Concha. 1995. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J. Neurovirol. 1:5-18. - PubMed
-
- Cinque, P., I. J. Koralnik, and D. B. Clifford. 2003. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J. Neurovirol. 9(Suppl. 1):88-92. - PubMed
-
- Clifford, D. B., and E. O. Major. 2001. The biology of JC virus and progressive multifocal leukoencephalopathy. J. Neurovirol. 4:279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

